AnnouncementsPage
2
2
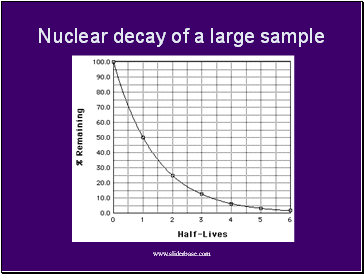
Rather, each isotope has its own “half-life,” the time in which a nucleus has a 50% chance of decaying.
Half-lives range from less than a millisecond (highly unstable isotopes) to billions of years (nearly stable).
+
Uranium-238
Thorium-234
Helium-4
Slide 11
Nuclear decay of a large sample
Slide 12
Radioactive Age Dating
A small percentage of all natural potassium is the radioactive isotope, potassium-40.
As a rock ages, its potassium-40 slowly disintegrates, leaving argon-40 atoms behind.
Argon is never incorporated into igneous crystals as they form, because it is a noble gas.
Therefore the ratio of argon-40 to potassium-40 is a direct measure of a rock’s age.
Possible problem: Heating a rock can allow trapped argon atoms to escape. If a rock has been heated, it might be older than we think it is.
Example: Potassium-40 decays to Argon-40 with a half-life of 1.4 billion years.
Slide 13
Radioactive Age Dates
Farmington Canyon Complex: 1.8 billion years
Oldest earth rocks: about 4 billion years
Oldest moon rocks: 4.6 billion years
Most meteorites: 4.6 billion years
Slide 14
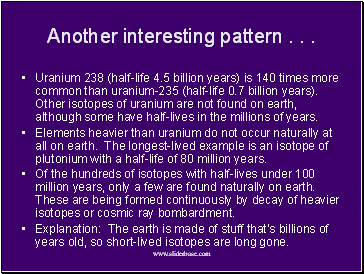
Another interesting pattern . . .
Uranium 238 (half-life 4.5 billion years) is 140 times more common than uranium-235 (half-life 0.7 billion years). Other isotopes of uranium are not found on earth, although some have half-lives in the millions of years.
Elements heavier than uranium do not occur naturally at all on earth. The longest-lived example is an isotope of plutonium with a half-life of 80 million years.
Of the hundreds of isotopes with half-lives under 100 million years, only a few are found naturally on earth. These are being formed continuously by decay of heavier isotopes or cosmic ray bombardment.
Explanation: The earth is made of stuff that’s billions of years old, so short-lived isotopes are long gone.
Slide 15
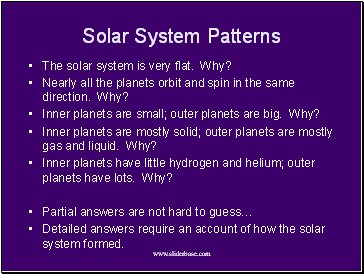
Solar System Patterns
The solar system is very flat. Why?
Nearly all the planets orbit and spin in the same direction. Why?
Inner planets are small; outer planets are big. Why?
Inner planets are mostly solid; outer planets are mostly gas and liquid. Why?
Inner planets have little hydrogen and helium; outer planets have lots. Why?
Partial answers are not hard to guess…
Detailed answers require an account of how the solar system formed.
Slide 16
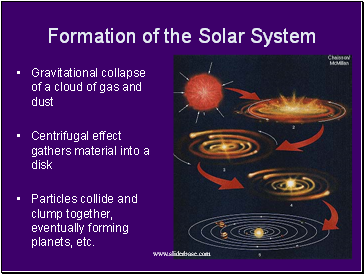
Formation of the Solar System
Contents
- Announcements
- Solar System Patterns
- How old is the solar system?
- Nuclear Isotopes
- Nuclear Decay (“Radioactivity”)
- Radioactive Age Dating
- Solar System Patterns
- Formation of the Solar System
- Success! >100 extrasolar planets discovered in last 12 years
Last added presentations
- Soil and Plant Nutrition
- Space Radiation
- Sound
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Sound
- Geophysical Concepts, Applications and Limitations
- Simulation at NASA for the Space Radiation Effort