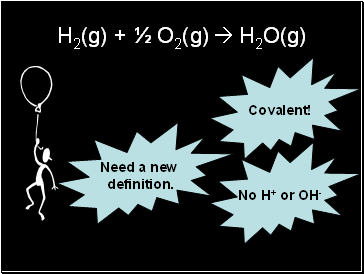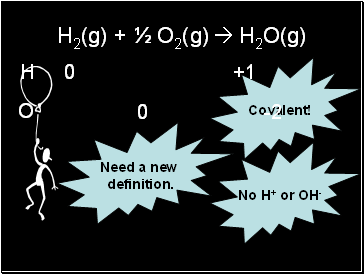Redox ReactionsPage
2
2
Oxidation Numbers
The oxidation numbers of atoms in an ion add up to the charge on the ion.
Oxidation state of S in SO42-?
? – 8 = -2
? = +6
Slide 14
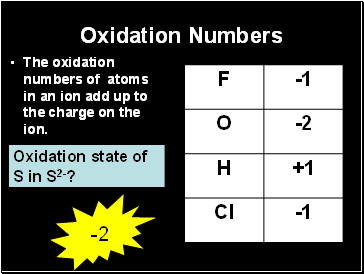
Oxidation Numbers
The oxidation numbers of atoms in an ion add up to the charge on the ion.
Oxidation state of S in S2-?
-2
Slide 15
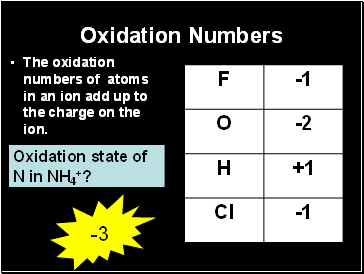
Oxidation Numbers
The oxidation numbers of atoms in an ion add up to the charge on the ion.
Oxidation state of N in NH4+?
-3
Slide 16
Try Question 2.
Slide 17
H2(g) + ½ O2(g) H2O(g)
Covalent!
No H+ or OH-
Need a new
definition.
Slide 18
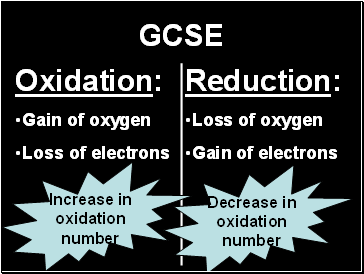
GCSE
Oxidation:
Gain of oxygen
Loss of electrons
Reduction:
Loss of oxygen
Gain of electrons
Increase in
oxidation
number
Decrease in
oxidation
number
Slide 19
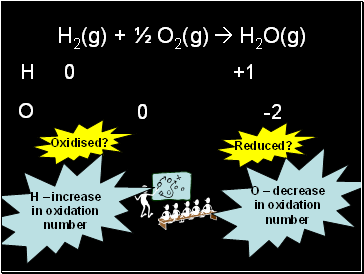
H2(g) + ½ O2(g) H2O(g)
Covalent!
No H+ or OH-
Need a new
definition.
+1
0
-2
0
O
H
Slide 20
H2(g) + ½ O2(g) H2O(g)
+1
0
-2
0
O
H
Oxidised?
H – increase
in oxidation
number
Reduced?
O – decrease
in oxidation
number
Slide 21
Try Question 3.
Slide 22
Oxidation Numbers and names
To avoid any confusion when an element can have several oxidation numbers, the oxidation number is usually mentioned in the compound’s name. In names like “elementate(X)”, the number refers to “element” and not the associated oxygens.
So if we look at some examples , we get the following names:-
KMnO4 potassium manganate(VII)
NaClO3 sodium chlorate(V)
POCl2F phosphorus(V) oxydichlorofluoride
NaH2PO3 sodium dihydrogenphosphate(III)
K2Cr2O7 potassium dichromate(VI)
Check the
numbers.
Slide 23
Try any 3 in Question 7.
Slide 24
Well done!
1 2
Contents
Last added presentations
- Practical Applications of Solar Energy
- Newton’s laws of motion
- Friction
- Solar Thermal Energy
- Soil and Plant Nutrition
- Upcoming Classes
- Radiation