The d BlockPage
2
2
Electrons from both inner d sub-shell and outer s sub-shell can be involved in compound formation
Slide 11
What is a transition metal?
Not all d block elements have incomplete d sub-shells
e.g. Zn has e.c. of [Ar]3d104s2, the Zn2+ ion ([Ar] 3d10) is not a typical TM ion
Similarly Sc forms Sc3+ which has the stable e.c of Ar. Sc3+ has no 3d electrons
Slide 12
What is a transition metal?
For this reason, a transition metal is defined as being an element which forms at least one ion with a partially filled sub-shell of d electrons.
In period 4 only Ti-Cu are TMís!
Note that when d block elements form ions the s electrons are lost first
Slide 13
What are TMís like?
TMís are metals
They are similar to each other but different from s block metals eg Na and Mg
Properties of TMís
Dense metals
Have high Tm and Tb
Tend to be hard and durable
Have high tensile strength
Have good mechanical properties
Slide 14
What are TMís like?
Properties derive from strong metallic bonding
TMís can release e- into the pool of mobile electrons from both outer and inner shells
Strong metallic bonds formed between the mobile pool and the +ve metal ions
Enables widespread use of TMs!
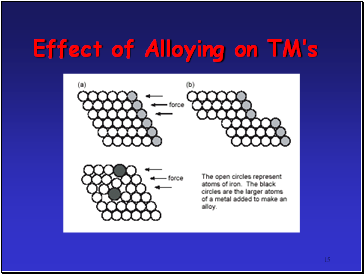
Alloys very important: inhibits slip in crystal lattice usually results in increased hardness and reduced malleability
Slide 15
Effect of Alloying on TMís
Slide 16
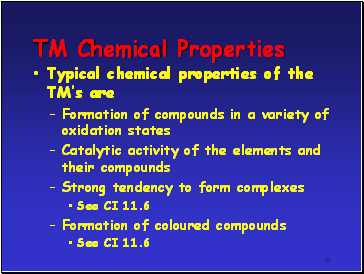
TM Chemical Properties
Typical chemical properties of the TMís are
Formation of compounds in a variety of oxidation states
Catalytic activity of the elements and their compounds
Strong tendency to form complexes
See CI 11.6
Formation of coloured compounds
See CI 11.6
Slide 17
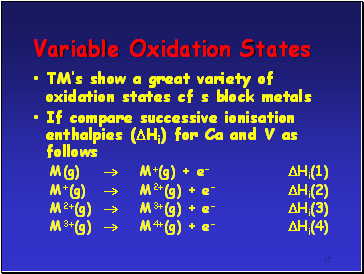
Variable Oxidation States
TMís show a great variety of oxidation states cf s block metals
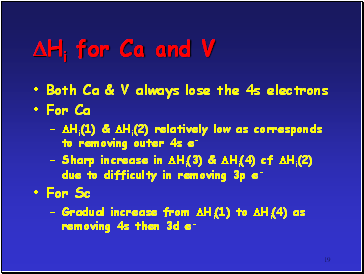
If compare successive ionisation enthalpies (Hi) for Ca and V as follows
M(g) M+(g) + e- Hi(1)
M+(g) M2+(g) + e- Hi(2)
M2+(g) M3+(g) + e- Hi(3)
M3+(g) M4+(g) + e- Hi(4)
Slide 18
18
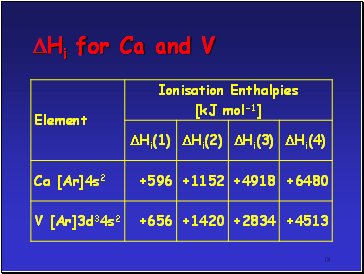
Hi for Ca and V
Slide 19
Contents
- The d block
- Electronic Configuration
- Chromium and Copper
- What is a transition metal?
- What are TMís like?
- Effect of Alloying on TMís
- TM Chemical Properties
- Variable Oxidation States
- Oxidation States of TMís
- Stability of OSís
- Catalytic Activity
- Heterogeneous Catalysis
- Suggested Mechanism
Last added presentations
- Practical Applications of Solar Energy
- Newtonís Laws of Motion
- Friction
- Sound
- Resource Acquisition and Transport in Vascular Plants
- Madame Marie Curie
- Magnetic field uses sound waves to ignite sun's ring of fire