The d BlockPage
3
3
19
Hi for Ca and V
Both Ca & V always lose the 4s electrons
For Ca
Hi(1) & Hi(2) relatively low as corresponds to removing outer 4s e-
Sharp increase in Hi(3) & Hi(4) cf Hi(2) due to difficulty in removing 3p e-
For Sc
Gradual increase from Hi(1) to Hi(4) as removing 4s then 3d e-
Slide 20
Oxidation States of TMís
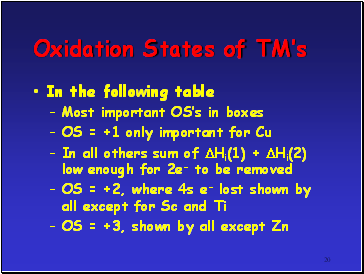
In the following table
Most important OSís in boxes
OS = +1 only important for Cu
In all others sum of Hi(1) + Hi(2) low enough for 2e- to be removed
OS = +2, where 4s e- lost shown by all except for Sc and Ti
OS = +3, shown by all except Zn
Slide 21
Oxidation States of TMís
Slide 22
Oxidation States of TMís
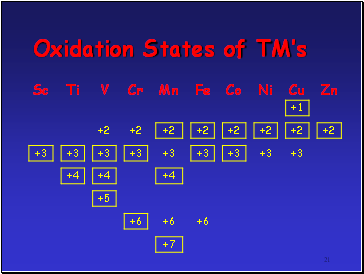
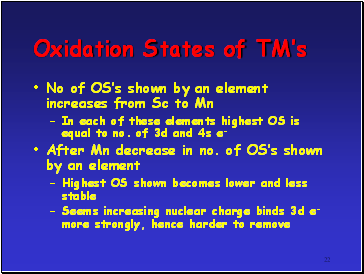
No of OSís shown by an element increases from Sc to Mn
In each of these elements highest OS is equal to no. of 3d and 4s e-
After Mn decrease in no. of OSís shown by an element
Highest OS shown becomes lower and less stable
Seems increasing nuclear charge binds 3d e- more strongly, hence harder to remove
Slide 23
Oxidation States of TMís
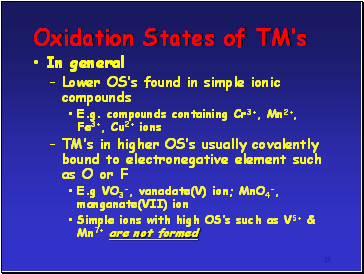
In general
Lower OSís found in simple ionic compounds
E.g. compounds containing Cr3+, Mn2+, Fe3+, Cu2+ ions
TMís in higher OSís usually covalently bound to electronegative element such as O or F
E.g VO3-, vanadate(V) ion; MnO4-, manganate(VII) ion
Simple ions with high OSís such as V5+ & Mn7+ are not formed
Slide 24
Stability of OSís
Change from one OS to another is a redox reaction
Relative stability of different OSís can be predicted by looking at Standard Electrode Potentials
E values
Slide 25
Stability of OSís
General trends
Higher OSís become less stable relative to lower ones on moving from left to right across the series
Compounds containing TMís in high OSís tend to be oxidising agents e.g MnO4-
Compounds with TMís in low OSís are often reducing agents e.g V2+ & Fe2+
Slide 26
Stability of OSís
General trends (continued)
Relative stability of +2 state with respect to +3 state increases across the series
For compounds early in the series, +2 state highly reducing
E.g. V2+(aq) & Cr2+(aq) strong reducing agents
Later in series +2 stable, +3 state highly oxidising
E.g. Co3+ is a strong oxidising agent, Ni3+ & Cu3+ do not exist in aqueous solution.
Contents
- The d block
- Electronic Configuration
- Chromium and Copper
- What is a transition metal?
- What are TMís like?
- Effect of Alloying on TMís
- TM Chemical Properties
- Variable Oxidation States
- Oxidation States of TMís
- Stability of OSís
- Catalytic Activity
- Heterogeneous Catalysis
- Suggested Mechanism
Last added presentations
- Newtonís laws of motion
- Soil and Plant Nutrition
- The Effects of Radiation on Living Things
- Heat-Energy on the Move
- Newtonís Laws of Motion
- Resource Acquisition and Transport in Vascular Plants
- Practical Applications of Solar Energy