Atoms IntroPage
3
3
Slide 17
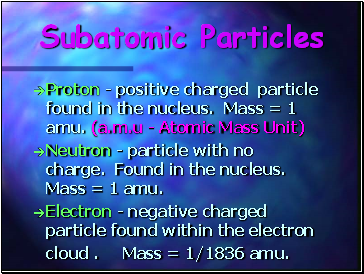
Subatomic Particles
Proton - positive charged particle found in the nucleus. Mass = 1 amu. (a.m.u - Atomic Mass Unit)
Neutron - particle with no charge. Found in the nucleus. Mass = 1 amu.
Electron - negative charged particle found within the electron cloud . Mass = 1/1836 amu.
Slide 18
Why are all Atoms are Electrically Neutral?
IMPORTANT
In all ATOMS the number of positively charged protons is always equal to the number of negatively charged electrons.
Normally in an atom the number of electrons within the electron cloud is equal to the number of protons in the nucleus. The positive and negative charges cancel each other out. Therefore, the atom is said to be electrically neutral.
If an atom gains or loses electrons the atom is no longer neutral . This can happen if the atom absorbs or releases energy The atom is then called an ION.
Slide 19
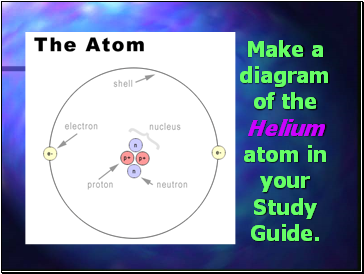
Make a diagram of the Helium atom in your Study Guide.
Slide 20
Now you are off to Jolly Old’ England!
Now you will visit a website from England to learn about Atomic Structure. Be sure to follow the instructions in your Study Guide.
Click here to visit a website at the BBC (British Broadcasting Company)
Slide 21
Isotopes
All atoms of an element have the same number of protons but the number of neutrons can vary. Atoms with the same number of protons and differing numbers of neutrons are called ISOTOPES.
Some Isotopes are unstable. The nucleus of unstable atoms do not hold together well. Radioactive decay is the process whereby the nucleus of unstable isotopes release fast moving particles and energy.
The discovery of Radioactivity almost happened by accident. Click on the picture of Henri Becquerel to learn about his discovery.
Slide 22
You have now completed the Atoms PowerPoint. See the teacher for further instructions.
Great Job!!
Contents
- Atoms
- Welcome to Atoms
- Atomic - Molecular Theory of Matter
- Matter
- Scientific Models
- What should a Model look like?
- Is this really an Atom?
- Indirect Evidence
- How can Indirect Evidence be Gathered?
- Can a Model be Changed?
- Where did it all begin?
- History of the Atom
- Atomic Structure
- Subatomic Particles
- Why are all Atoms are Electrically Neutral?
- Now you are off to Jolly Old’ England!
- Isotopes
Last added presentations
- Solar Energy
- Direct heat utilization of geothermal energy
- Ch 9 Nuclear Radiation
- Newton’s laws of motion
- Mechanics Lecture
- Upcoming Classes
- Radiation Safety and Operations