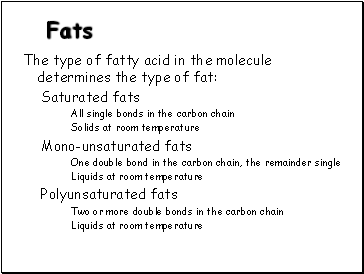Basic Biochemistry - Carbohydrate, Protein and FatPage
2
2
Because water is lost, the process is called:
Condensation synthesis, or
Condensation polymerization
Slide 12
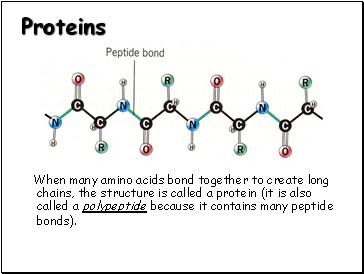
Proteins
When many amino acids bond together to create long chains, the structure is called a protein (it is also called a polypeptide because it contains many peptide bonds).
Slide 13
Proteins
Proteins are large molecules that may consist of hundreds, or even thousands of amino acids.
While there are hundreds of thousands of different proteins that exist in nature, they are all made up of different combinations of amino acids.
Slide 14
Insulin
Slide 15
Fats
Fats are a sub-group of compounds known as lipids that are found in the body and have the general property of being hydrophobic (meaning they are insoluble in water).
Other lipids include waxes, and steroids, such as cholesterol.
Slide 16
Fats
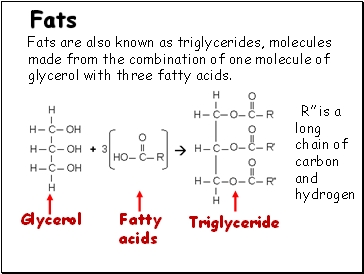
Fats are also known as triglycerides, molecules made from the combination of one molecule of glycerol with three fatty acids.
Glycerol
Fatty
acids
Triglyceride
R is a long chain of carbon and hydrogen
Slide 17
Fats
The type of fatty acid in the molecule determines the type of fat:
Saturated fats
All single bonds in the carbon chain
Solids at room temperature
Mono-unsaturated fats
One double bond in the carbon chain, the remainder single
Liquids at room temperature
Polyunsaturated fats
Two or more double bonds in the carbon chain
Liquids at room temperature
Slide 18
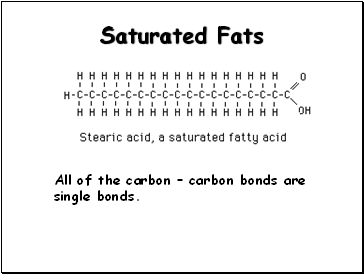
Saturated Fats
All of the carbon carbon bonds are single bonds.
Slide 19
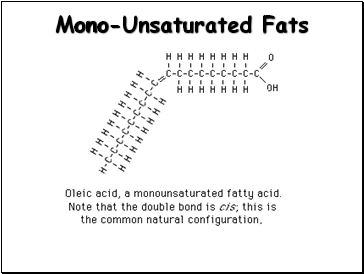
Mono-Unsaturated Fats
Slide 20
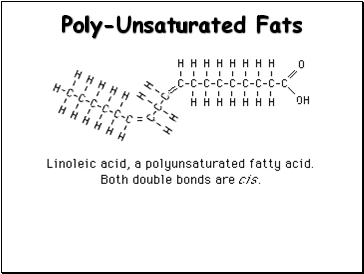
Poly-Unsaturated Fats
1 2
Contents
- Biochemistry
- Carbohydrates
- Simple Sugars
- Complex Carbohydrates
- Structure of Glycogen
- Proteins
- Insulin
- Fats
- Saturated Fats
- Mono-Unsaturated Fats
- Poly-Unsaturated Fats
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Resource Acquisition and Transport in Vascular Plants
- Madame Marie Curie
- Radiation
- Magnetic field uses sound waves to ignite sun's ring of fire
- Newton's Laws
- Buoyancy