Chemical Bonding revisedPage
3
3
Slide 19
Slide 20
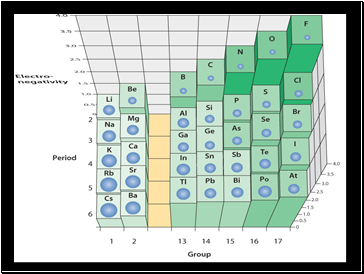
Electronegativity
For main group elements, electronegativity tends to increase with the group number (left to right on the periodic table).
Notice the noble gases do not have electronegativities, why is that?
Electronegativities also increase as you move vertically up a group number.
Slide 21
Atom Size
For any period in the periodic table, as you move right the size of the atom decreases
Why does this trend exist?
Slide 22
Atom size
As the number of protons increases, the force attracting the electrons increases
The electrons are pulled closer to the nucleus of the atom
Slide 23
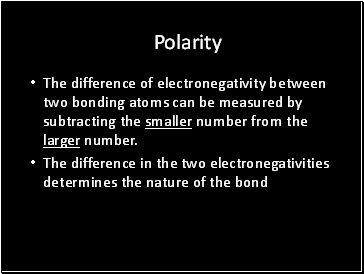
Polarity
The difference of electronegativity between two bonding atoms can be measured by subtracting the smaller number from the larger number.
The difference in the two electronegativities determines the nature of the bond
Slide 24
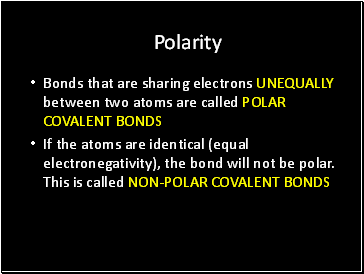
Polarity
Bonds that are sharing electrons UNEQUALLY between two atoms are called POLAR COVALENT BONDS
If the atoms are identical (equal electronegativity), the bond will not be polar. This is called NON-POLAR COVALENT BONDS
Slide 25
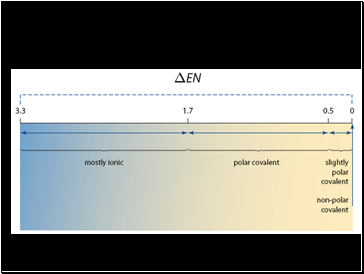
Polar covalent bonds have a positive “pole” and a negative “pole” so they are also referred to as “bond dipoles”
Polar covalent bonds have an electronegativity difference between 0 and 1.7
Ionic bonds have an electronegativity difference between 1.7 and 3.3
Slide 26
Slide 27
Polar Molecules
Are molecules that contain polar bonds necessarily polar?
Examples of H2O and CO2
To determine if a molecule is polar we need to look at the overall direction of polarity
Draw in polarity arrows on your molecule and determine if the molecules are polar or not
Slide 28
Ionic Bonds
Ionic bonds form from the electrostatic attraction between oppositely charged ions
Atoms become ionic by losing or gaining electrons from the atom it is bonding with
Remember that an atom will lose its electrons to fill its outer level if its valence level is less than half full, as it is with metals
Slide 29
Contents
- Covalent Bonding
- Lewis Dot Diagrams
- Multiple Bonds
- Multiple Lewis Structures
- Structural Diagrams
- Lewis Dot Diagram Worksheet
- Stereochemistry – The Structures of Molecular Compounds
- Linear
- Trigonal Planar
- Tetrahedral
- Steps to Predicting Molecular Shapes
- Electronegativity
- Atom Size
- Polarity
- Polar Molecules
- Ionic Bonds
- Metallic Bonding
- Ionic Crystals
- Crystal formation
- Network Solids
- Intermolecular Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Hydrogen Bonding in Water
- Hydrogen Bonds in Ice
- Unique Properties Reading
- London Dispersion Forces
- Factors Affecting Magnitude
- Structures and Properties of Compounds
- Time of Hydrogen Bonding
- Melting and Boiling Points
- Molecular Forces
- Mechanical Properties of Solids
- Conductivity
Last added presentations
- Newton’s Laws of Motion
- Radioactivity and Nuclear Reactions
- Sound
- Heat-Energy on the Move
- Sensory and Motor Mechanisms
- Newton’s Law of Gravity
- Buoyancy