Chemical Bonding revisedPage
6
6
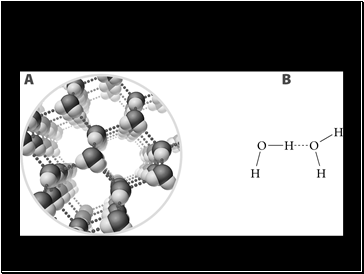
Each water molecule is hydrogen bonded to four other water molecules
The water molecules in ice are farther apart than in liquid water, therefore ice is less dense than liquid water**
Hydrogen bonds are the strongest in the form shown in the next diagram
Slide 52
Slide 53
Unique Properties Reading
Read the handout on the unique properties of water
Slide 54
London Dispersion Forces
Dispersion forces act between all molecules, but in non-polar molecules they are the only force
Even though there are no permanent dipoles in non-polar molecules, it is possible to induce dipoles
Slide 55
Slide 56
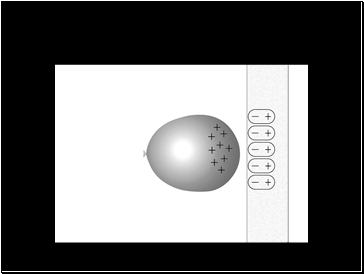
Non-polar molecules also spontaneously form temporary dipoles
Electrons are in constant, rapid motion
For a brief moment the electron distribution can be uneven
This can form a positive pole and a negative pole in the molecule
Slide 57
The temporary dipole in the molecule can induce a temporary dipole in the next molecule, like the balloon and the wall
The process disperses through the substance
Slide 58
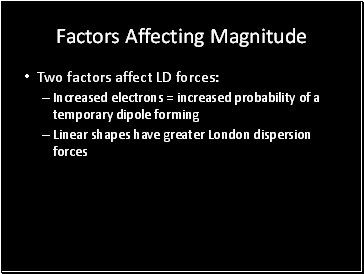
Factors Affecting Magnitude
Two factors affect LD forces:
Increased electrons = increased probability of a temporary dipole forming
Linear shapes have greater London dispersion forces
Slide 59
Structures and Properties of Compounds
The state of a substance (solid, liquid or gas) depends on the strength of the intermolecular forces
As particles gain kinetic energy (heat) they break their intermolecular bonds and change state
Slide 60
Time of Hydrogen Bonding
FYI – hydrogen bonds in liquid water break and reform 100 000 000 000 (1011) times every second
Slide 61
Melting and Boiling Points
Melting and boiling points of ionic substances and metals are about the same magnitude
Melting and boiling points of molecular substances are much lower
What does that tell us about the forces?
Slide 62
Ionic bonds are much stronger if the ions have a large charge
Contents
- Covalent Bonding
- Lewis Dot Diagrams
- Multiple Bonds
- Multiple Lewis Structures
- Structural Diagrams
- Lewis Dot Diagram Worksheet
- Stereochemistry – The Structures of Molecular Compounds
- Linear
- Trigonal Planar
- Tetrahedral
- Steps to Predicting Molecular Shapes
- Electronegativity
- Atom Size
- Polarity
- Polar Molecules
- Ionic Bonds
- Metallic Bonding
- Ionic Crystals
- Crystal formation
- Network Solids
- Intermolecular Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Hydrogen Bonding in Water
- Hydrogen Bonds in Ice
- Unique Properties Reading
- London Dispersion Forces
- Factors Affecting Magnitude
- Structures and Properties of Compounds
- Time of Hydrogen Bonding
- Melting and Boiling Points
- Molecular Forces
- Mechanical Properties of Solids
- Conductivity
Last added presentations
- Mechanics Lecture
- Radiation
- Buoyancy
- Ch 9 Nuclear Radiation
- Radioactivity and Nuclear Reactions
- Simulation at NASA for the Space Radiation Effort
- Resource Acquisition and Transport in Vascular Plants