Chemical BondsPage
5
5
Group VIIA – accept one e- to form –1 (Cl-1)
Slide 34
Naming IONIC compounds
Anions – suffix – “ide”
So Cl- is chloride
Oxygen O2- is OXIDE
S2- is SULFIDE
Cations
For Na+, Ca2+, the name of the ion is the same except refer to the ion.
So SODIUM ION or SODIUM CATION
NaCl - sodium chloride
CaCl2 - calcium chloride
Slide 35
Covalent, charged compounds - MOLECULAR IONS
Positive Molecular Ions
End the name with “ium” or “onium”
NH4+ is ammonium, H3O + is hydronium
Negative Molecular Ions
Slide 36
The transition elements are chemically quite different from the metals in the “A” block, due to differences in electronic configuration
For example, Fe can loose two or three electrons to become Fe2+ and Fe3+, respectively.
Transition Elements
Slide 37
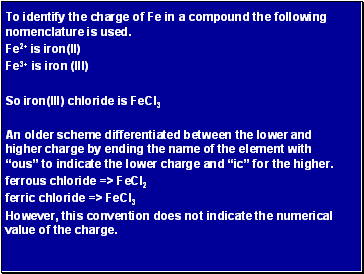
To identify the charge of Fe in a compound the following nomenclature is used.
Fe2+ is iron(II)
Fe3+ is iron (III)
So iron(III) chloride is FeCl3
An older scheme differentiated between the lower and higher charge by ending the name of the element with “ous” to indicate the lower charge and “ic” for the higher.
ferrous chloride => FeCl2
ferric chloride => FeCl3
However, this convention does not indicate the numerical value of the charge.
Contents
Last added presentations
- Radiation Safety and Operations
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Sensory and Motor Mechanisms
- Newton's laws of motion
- Health Physics
- Newton’s law of universal gravitation
- Heat-Energy on the Move