Chemical BondsPage
4
4
TABLE
Slide 27
Generally,
Metallic compound + nonmetallic compound IONIC compound
Ionic compounds are generally high-melting solids that are good conductors of heat and electricity in the molten state.
Examples are NaCl, common salt, and NaF, sodium fluoride.
TABLE
Slide 28
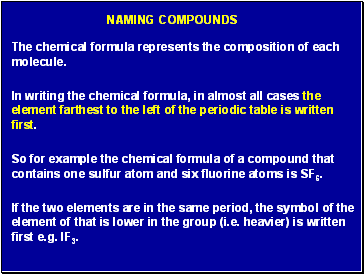
Naming compounds
The chemical formula represents the composition of each molecule.
In writing the chemical formula, in almost all cases the element farthest to the left of the periodic table is written first.
So for example the chemical formula of a compound that contains one sulfur atom and six fluorine atoms is SF6.
If the two elements are in the same period, the symbol of the element of that is lower in the group (i.e. heavier) is written first e.g. IF3.
Slide 29
In naming covalent compounds, the name of the first element in the formula is unchanged.
The suffix -ide is added to the second element.
Often a prefix to the name of the second element indicates the number of the element in the compound
SF6 sulfur hexafluoride
P4O10 tetraphosphorous decoxide
CO carbon monoxide
CO2 carbon dioxide
Slide 30
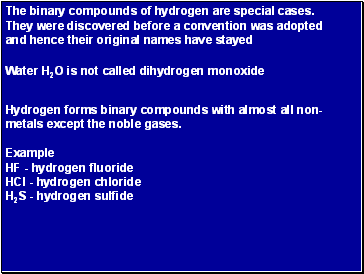
The binary compounds of hydrogen are special cases. They were discovered before a convention was adopted and hence their original names have stayed
Hydrogen forms binary compounds with almost all non-metals except the noble gases.
Example
HF - hydrogen fluoride
HCl - hydrogen chloride
H2S - hydrogen sulfide
Water H2O is not called dihydrogen monoxide
Slide 31
Organic molecules (containing C) have a separate nomenclature
The molecular formulas for compounds containing C and H (called hydrocarbons) are written with C first. Example, CH4, C2H6, etc.
Slide 32
Binary ionic compounds
Compounds formed by elements on opposite sides of the periodic table which either give up (left side) or take up electrons (right side).
Depending on the atom, there can be an exchange of more than one electron resulting in charges greater than ±1.
Slide 33
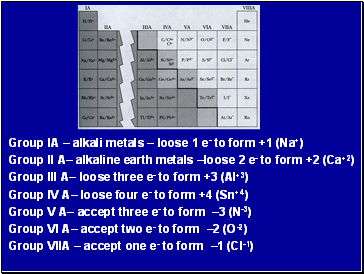
Group IA alkali metals loose 1 e- to form +1 (Na+)
Group II A alkaline earth metals loose 2 e- to form +2 (Ca+2)
Group III A loose three e- to form +3 (Al+3)
Group IV A loose four e- to form +4 (Sn+4)
Group V A accept three e- to form 3 (N-3)
Group VI A accept two e- to form 2 (O-2)
Contents
Last added presentations
- Newtons Laws of Motion
- Radiation
- Sound
- Soil and Plant Nutrition
- The Effects of Radiation on Living Things
- Resource Acquisition and Transport in Vascular Plants
- Newton's laws of motion