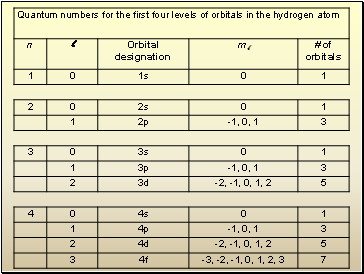
Electron Quantum NumbersPage
2
2
Shape of f (l = 3) orbitals
Slide 11
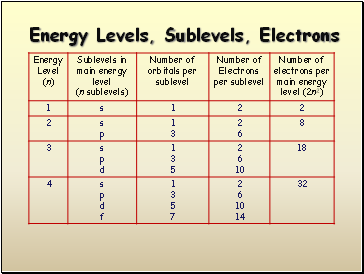
Energy Levels, Sublevels, Electrons
Slide 12
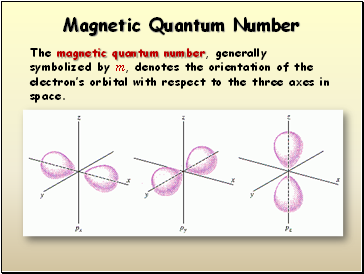
Magnetic Quantum Number
The magnetic quantum number, generally symbolized by m, denotes the orientation of the electron’s orbital with respect to the three axes in space.
Slide 13
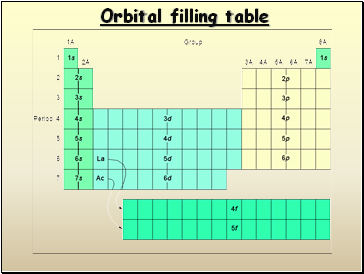
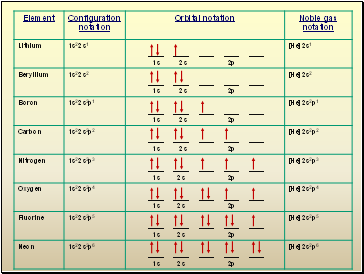
Orbital filling table
Slide 14
Electron Spin
The Spin Quantum Number describes the behavior (direction of spin) of an electron within a magnetic field.
Possibilities for electron spin:
Slide 15
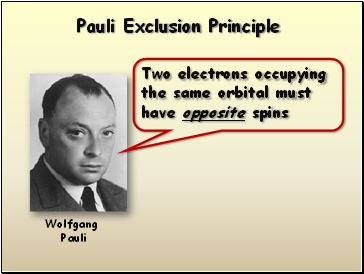
Pauli Exclusion Principle
Two electrons occupying the same orbital must have opposite spins
Wolfgang
Pauli
Slide 16
Slide 17
Exclusion Warning!
Assigning quantum numbers to electrons has been exluded and will not be tested!
The following slides are purely for your entertainment
Slide 18
Assigning the Numbers
The three quantum numbers (n, l, and m) are integers.
The principal quantum number (n) cannot be zero.
n must be 1, 2, 3, etc.
The angular momentum quantum number (l ) can be any integer between 0 and n - 1.
For n = 3, l can be either 0, 1, or 2.
The magnetic quantum number (ml) can be any integer between -l and +l.
For l = 2, m can be either -2, -1, 0, +1, +2.
Slide 19
1 2
Contents
- Electron Orbitals
- Quantum Mechanical Model of the Atom
- Heisenberg Uncertainty Principle
- Quantum Numbers
- Electron Energy Level (Shell)
- Electron Orbitals
- Orbital shape
- Energy Levels, Sublevels, Electrons
- Magnetic Quantum Number
- Orbital filling table
- Electron Spin
- Pauli Exclusion Principle
- Exclusion Warning!
- Assigning the Numbers
Last added presentations
- Newton’s third law of motion
- Madame Marie Curie
- Sound
- Magnetic field uses sound waves to ignite sun's ring of fire
- Gravitation
- Simulation at NASA for the Space Radiation Effort
- Direct heat utilization of geothermal energy