Atomic Structure and PeriodicityPage
1
1
Slide 1
Chapter 7 Atomic Structure and Periodicity
Electromagnetic Radiation
Radiant energy that exhibits wavelength-like behavior and travels through space at the speed of light in a vacuum.
Example: The sun light, energy used in microwave oven, the x-rays used by doctors.
Slide 2
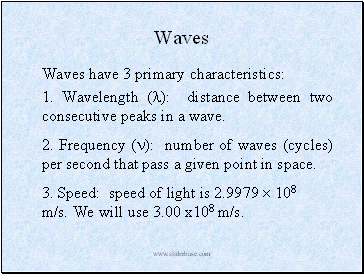
Waves
Waves have 3 primary characteristics:
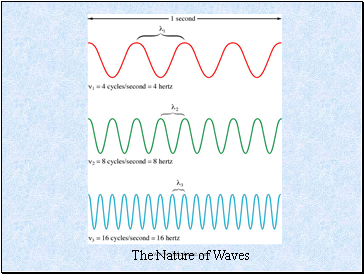
1. Wavelength (): distance between two consecutive peaks in a wave.
2. Frequency (): number of waves (cycles) per second that pass a given point in space.
3. Speed: speed of light is 2.9979 108 m/s. We will use 3.00 x108 m/s.
Slide 3
The Nature of Waves
Slide 4
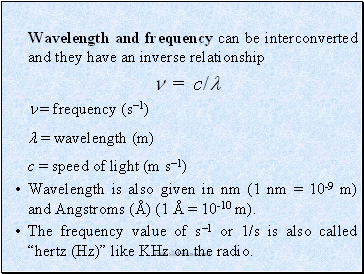
Wavelength and frequency can be interconverted and they have an inverse relationship
= c/
= frequency (s1)
= wavelength (m)
c = speed of light (m s1)
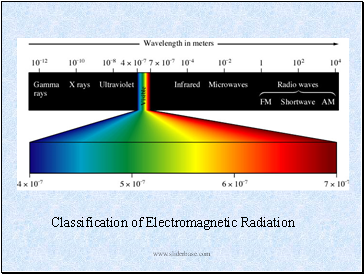
Wavelength is also given in nm (1 nm = 10-9 m) and Angstroms (Å) (1 Å = 10-10 m).
The frequency value of s1 or 1/s is also called “hertz (Hz)” like KHz on the radio.
Slide 5
Classification of Electromagnetic Radiation
Slide 6
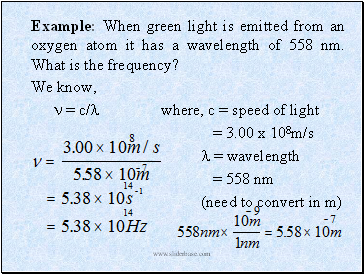
Example: When green light is emitted from an oxygen atom it has a wavelength of 558 nm. What is the frequency?
We know,
= c/ where, c = speed of light
= 3.00 x 108m/s
= wavelength
= 558 nm
(need to convert in m)
Slide 7
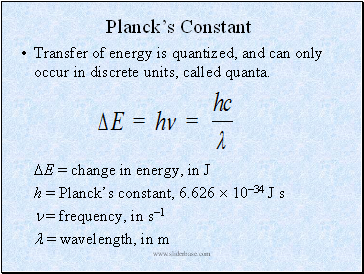
Planck’s Constant
Transfer of energy is quantized, and can only occur in discrete units, called quanta.
E = change in energy, in J
h = Planck’s constant, 6.626 1034 J s
= frequency, in s1
= wavelength, in m
Slide 8
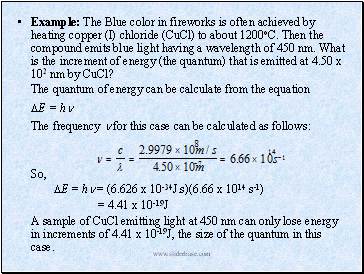
Example: The Blue color in fireworks is often achieved by heating copper (I) chloride (CuCl) to about 1200oC. Then the compound emits blue light having a wavelength of 450 nm. What is the increment of energy (the quantum) that is emitted at 4.50 x 102 nm by CuCl?
The quantum of energy can be calculate from the equation
E = h
The frequency for this case can be calculated as follows:
So,
E = h = (6.626 x 10-34J.s)(6.66 x 1014 s-1)
= 4.41 x 10-19J
Contents
- Waves
- Planck’s Constant
- Energy and Mass
- Wavelength and Mass
- Atomic Spectrum of Hydrogen
- The Bohr Model
- Quantum Mechanics
- Heisenberg Uncertainty Principle
- Quantum Numbers (QN)
- Orbital Shapes and Energies
- Representation of p orbitals
- Representation d orbitals
- Energy Diagram for Hydrogen Atom
- Pauli Exclusion Principle
- Polyelectronic Atoms
- Aufbau Principle
- Hund’s Rule
- Valence Electrons
- Broad Periodic Table Classifications
- Ionization Energy
- Periodic Trends
- Electron Affinity
- Periodic Trends
- Information Contained in the Periodic Table
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Geophysical Concepts, Applications and Limitations
- Madame Marie Curie
- Radiation
- Simulation at NASA for the Space Radiation Effort
- Buoyancy
- Radiation Safety and Operations