Polar Covalent Bonds Acids and BasesPage
3
3
16
Rules for Resonance Forms
Individual resonance forms are imaginary - the real structure is a hybrid (only by knowing the contributors can you visualize the actual structure)
Resonance forms differ only in the placement of their or nonbonding electrons
Different resonance forms of a substance don’t have to be equivalent
Resonance forms must be valid Lewis structures: the octet rule applies
The resonance hybrid is more stable than any individual resonance form would be
Slide 17
17
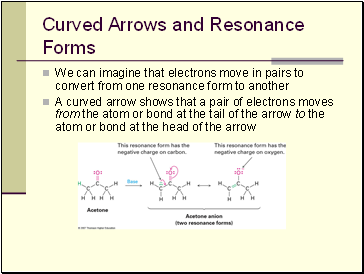
Curved Arrows and Resonance Forms
We can imagine that electrons move in pairs to convert from one resonance form to another
A curved arrow shows that a pair of electrons moves from the atom or bond at the tail of the arrow to the atom or bond at the head of the arrow
Slide 18
18
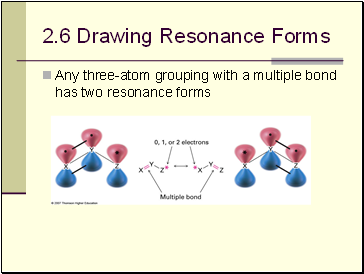
Drawing Resonance Forms
Any three-atom grouping with a multiple bond has two resonance forms
Slide 19
19
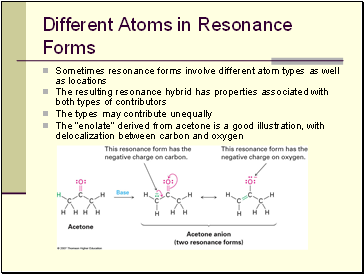
Different Atoms in Resonance Forms
Sometimes resonance forms involve different atom types as well as locations
The resulting resonance hybrid has properties associated with both types of contributors
The types may contribute unequally
The “enolate” derived from acetone is a good illustration, with delocalization between carbon and oxygen
Slide 20
20
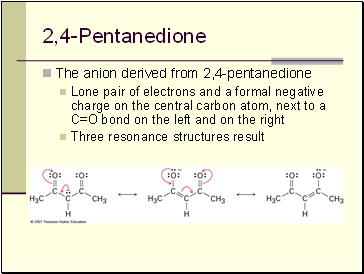
Pentanedione
The anion derived from 2,4-pentanedione
Lone pair of electrons and a formal negative charge on the central carbon atom, next to a C=O bond on the left and on the right
Three resonance structures result
Slide 21
21
Acids and Bases: The Brønsted–Lowry Definition
The terms “acid” and “base” can have different meanings in different contexts
For that reason, we specify the usage with more complete terminology
The idea that acids are solutions containing a lot of “H+” and bases are solutions containing a lot of “OH-” is not very useful in organic chemistry
Instead, Brønsted–Lowry theory defines acids and bases by their role in reactions that transfer protons (H+) between donors and acceptors
Slide 22
22
Brønsted Acids and Bases
“Brønsted-Lowry” is usually shortened to “Brønsted”
A Brønsted acid is a substance that donates a hydrogen ion (H+)
A Brønsted base is a substance that accepts the H+
“proton” is a synonym for H+ - loss of an electron from H leaving the bare nucleus—a proton
Contents
- Why this chapter?
- Polar Covalent Bonds: Electronegativity
- Bond Polarity and Electronegativity
- Electrostatic Potential Maps
- Polar Covalent Bonds: Dipole Moments
- Formal Charges
- Resonance
- Rules for Resonance Forms
- Drawing Resonance Forms
- Pentanedione
- Acids and Bases: The Brønsted–Lowry Definition
- Acid and Base Strength
- Predicting Acid–Base Reactions from pKa Values
- Organic Acids and Organic Bases
- Acids and Bases: The Lewis Definition
- Molecular Models
- Noncovalent Interactions
Last added presentations
- Buoyancy
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Newton's Laws
- Motion
- Newton’s third law of motion
- Direct heat utilization of geothermal energy
- Newton's laws of motion