Polar Covalent Bonds Acids and BasesPage
5
5
31
Organic Bases
Have an atom with a lone pair of electrons that can bond to H+
Nitrogen-containing compounds derived from ammonia are the most common organic bases
Oxygen-containing compounds can react as bases when with a strong acid or as acids with strong bases
Slide 32
32
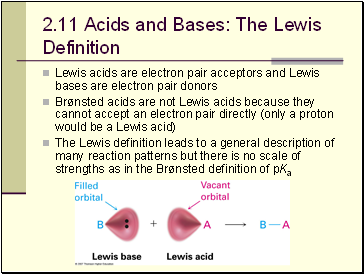
Acids and Bases: The Lewis Definition
Lewis acids are electron pair acceptors and Lewis bases are electron pair donors
Brønsted acids are not Lewis acids because they cannot accept an electron pair directly (only a proton would be a Lewis acid)
The Lewis definition leads to a general description of many reaction patterns but there is no scale of strengths as in the Brønsted definition of pKa
Slide 33
33
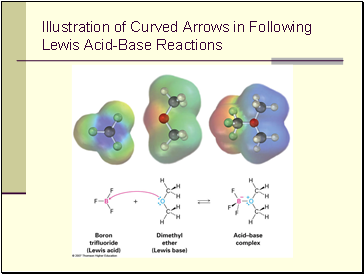
Lewis Acids and the Curved Arrow Formalism
The Lewis definition of acidity includes metal cations, such as Mg2+
They accept a pair of electrons when they form a bond to a base
Group 3A elements, such as BF3 and AlCl3, are Lewis acids because they have unfilled valence orbitals and can accept electron pairs from Lewis bases
Transition-metal compounds, such as TiCl4, FeCl3, ZnCl2, and SnCl4, are Lewis acids
Organic compounds that undergo addition reactions with Lewis bases (discussed later) are called electrophiles and therefore Lewis Acids
The combination of a Lewis acid and a Lewis base can shown with a curved arrow from base to acid
Slide 34
34
Illustration of Curved Arrows in Following Lewis Acid-Base Reactions
Slide 35
35
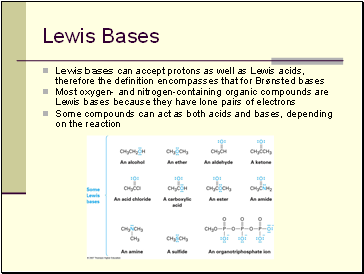
Lewis Bases
Lewis bases can accept protons as well as Lewis acids, therefore the definition encompasses that for Brønsted bases
Most oxygen- and nitrogen-containing organic compounds are Lewis bases because they have lone pairs of electrons
Some compounds can act as both acids and bases, depending on the reaction
Slide 36
36
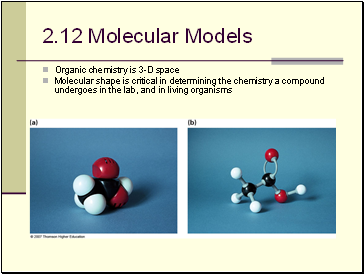
Molecular Models
Organic chemistry is 3-D space
Molecular shape is critical in determining the chemistry a compound undergoes in the lab, and in living organisms
Slide 37
37
Noncovalent Interactions
Several types:
Dipole-dipole forces
Dispersion forces
Hydrogen bonds
Slide 38
38
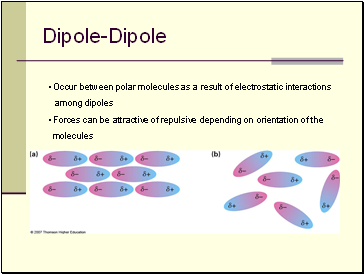
Dipole-Dipole
• Occur between polar molecules as a result of electrostatic interactions
among dipoles
• Forces can be attractive of repulsive depending on orientation of the
Contents
- Why this chapter?
- Polar Covalent Bonds: Electronegativity
- Bond Polarity and Electronegativity
- Electrostatic Potential Maps
- Polar Covalent Bonds: Dipole Moments
- Formal Charges
- Resonance
- Rules for Resonance Forms
- Drawing Resonance Forms
- Pentanedione
- Acids and Bases: The Brønsted–Lowry Definition
- Acid and Base Strength
- Predicting Acid–Base Reactions from pKa Values
- Organic Acids and Organic Bases
- Acids and Bases: The Lewis Definition
- Molecular Models
- Noncovalent Interactions
Last added presentations
- Newton’s Laws of Motion
- Upcoming Classes
- Sound
- Mechanics Lecture
- Newton’s law of universal gravitation
- Resource Acquisition and Transport in Vascular Plants
- Buoyancy