PH CalculationsPage
1
1
Slide 1
pH Calculations
Soren Sorensen
Slide 2
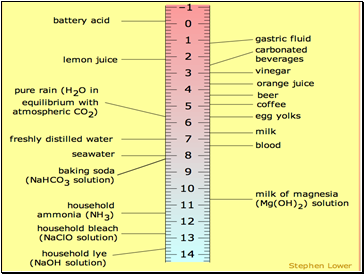
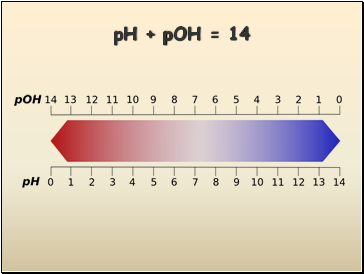
pH Scale
Slide 3
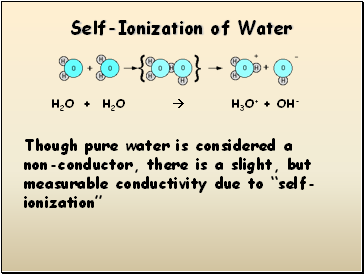
Self-Ionization of Water
H2O + H2O H3O+ + OH-
Though pure water is considered a non-conductor, there is a slight, but measurable conductivity due to “self-ionization”
Slide 4
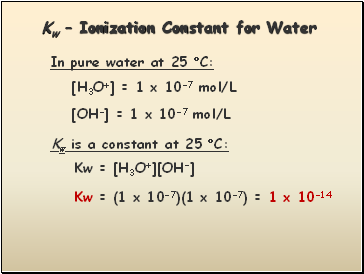
Kw – Ionization Constant for Water
In pure water at 25 C:
[H3O+] = 1 x 10-7 mol/L
[OH-] = 1 x 10-7 mol/L
Kw is a constant at 25 C:
Kw = [H3O+][OH-]
Kw = (1 x 10-7)(1 x 10-7) = 1 x 10-14
Slide 5
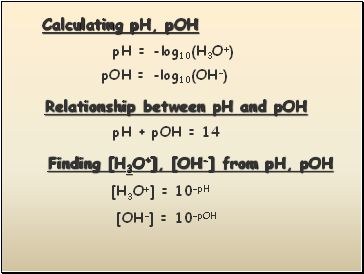
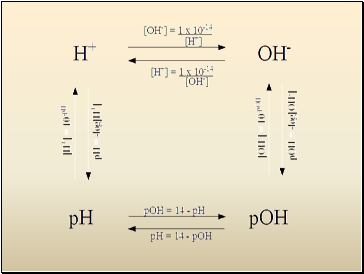
Calculating pH, pOH
pH = -log10(H3O+)
pOH = -log10(OH-)
Relationship between pH and pOH
pH + pOH = 14
Finding [H3O+], [OH-] from pH, pOH
[H3O+] = 10-pH
[OH-] = 10-pOH
Slide 6
pH + pOH = 14
Slide 7
Contents
Last added presentations
- Mechanics Lecture
- Health Physics
- Newton's laws of motion
- Direct heat utilization of geothermal energy
- Magnetic field uses sound waves to ignite sun's ring of fire
- Newton’s third law of motion
- Understanding Heat Transfer, Conduction, Convection and Radiation
© 2010-2026 powerpoint presentations