Atoms, molecules and ionsPage
2
2
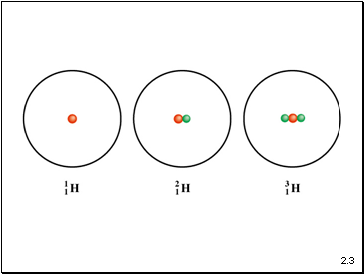
Atomic number (Z) = number of protons in nucleus
Mass number (A) = number of protons + number of neutrons
= atomic number (Z) + number of neutrons
Isotopes are atoms of the same element (X) with different numbers of neutrons in the nucleus
2.3
Slide 14
2.3
Slide 15
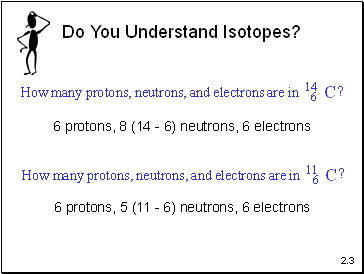
6 protons, 8 (14 - 6) neutrons, 6 electrons
6 protons, 5 (11 - 6) neutrons, 6 electrons
Do You Understand Isotopes?
2.3
Slide 16
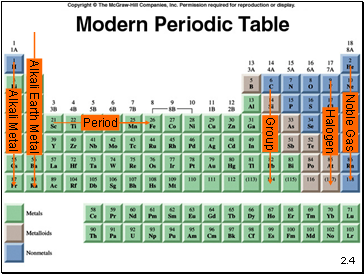
2.4
Slide 17
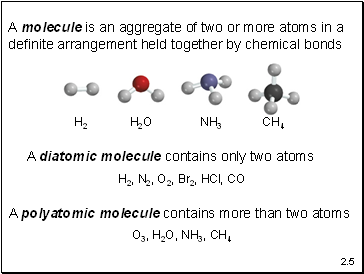
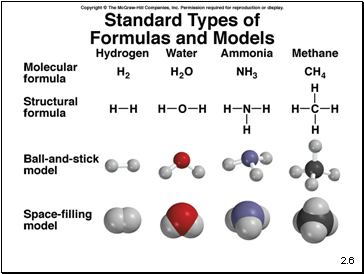
A molecule is an aggregate of two or more atoms in a definite arrangement held together by chemical bonds
A diatomic molecule contains only two atoms
H2, N2, O2, Br2, HCl, CO
A polyatomic molecule contains more than two atoms
O3, H2O, NH3, CH4
2.5
Slide 18
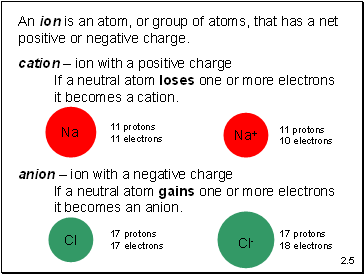
An ion is an atom, or group of atoms, that has a net positive or negative charge.
cation – ion with a positive charge
If a neutral atom loses one or more electrons
it becomes a cation.
anion – ion with a negative charge
If a neutral atom gains one or more electrons
it becomes an anion.
2.5
Slide 19
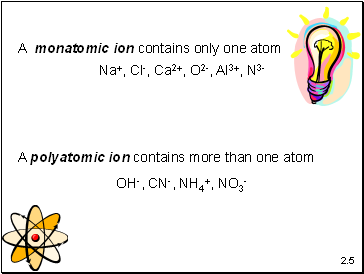
A monatomic ion contains only one atom
A polyatomic ion contains more than one atom
2.5
Na+, Cl-, Ca2+, O2-, Al3+, N3-
OH-, CN-, NH4+, NO3-
Slide 20
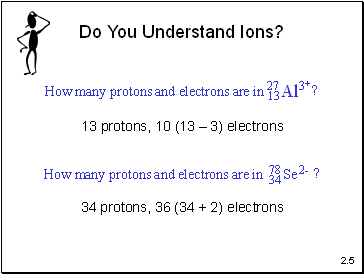
13 protons, 10 (13 – 3) electrons
34 protons, 36 (34 + 2) electrons
Do You Understand Ions?
2.5
Slide 21
2.5
Slide 22
2.6
Slide 23
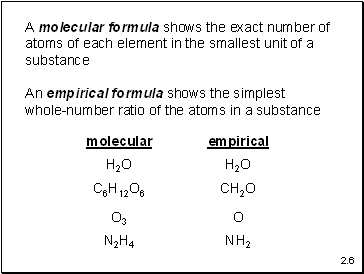
A molecular formula shows the exact number of atoms of each element in the smallest unit of a substance
An empirical formula shows the simplest
whole-number ratio of the atoms in a substance
H2O
C6H12O6
CH2O
O3
O
N2H4
NH2
2.6
Slide 24
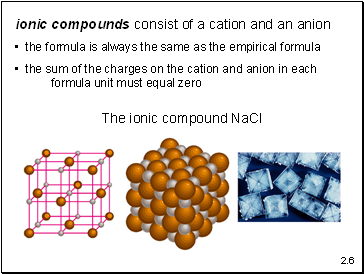
ionic compounds consist of a cation and an anion
the formula is always the same as the empirical formula
the sum of the charges on the cation and anion in each formula unit must equal zero
The ionic compound NaCl
2.6
Slide 25
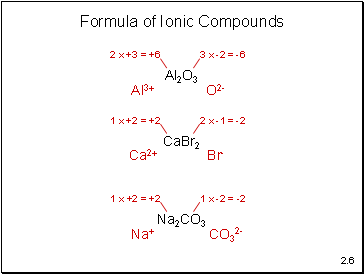
Formula of Ionic Compounds
Contents
- Atoms, Molecules and Ions
- Dalton’s Atomic Theory (1808)
- Chadwick’s Experiment (1932)
- Formula of Ionic Compounds
- Chemical Nomenclature
Last added presentations
- Radiation Safety and Operations
- Health Physics
- Sound
- Geophysical Concepts, Applications and Limitations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Madame Marie Curie
- Direct heat utilization of geothermal energy