Rutherford ScatteringPage
1
1
Slide 1
Nuclear Reactions
Rutherford’s Alpha Scattering Experiment
Slide 2
CS 4.1
Describe how Rutherford showed that:
(a) The nucleus had a relatively small diameter compared with that of the atom.
(b) Most of the mass of the atom is concentrated in the nucleus.
Slide 3
In the beginning……
In the early days of atomic theory, many physicists tried to explain the model of an atom.
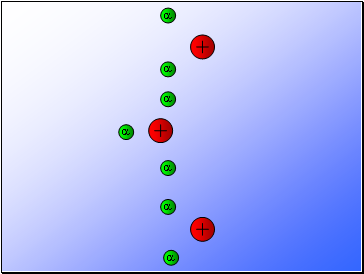
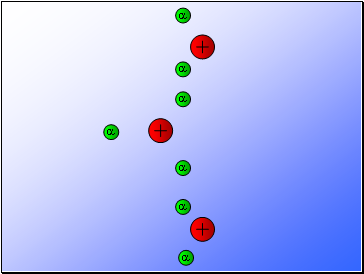
In 1902, Ernest Rutherford showed that alpha particles emitted from the decay of unstable radioactive materials were electrically charged helium nuclei travelling at high speed.
In 1909, Rutherford used alpha particles to investigate the composition of gold foil (i.e. to explain the model of an atom).
Slide 4
Aim
To investigate the composition of gold foil using alpha particles (i.e. to explain the model of an atom).
Slide 5
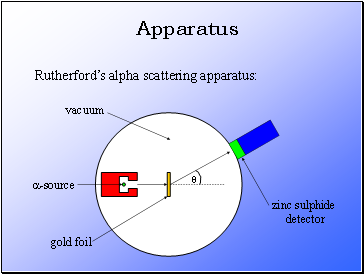
Apparatus
Rutherford’s alpha scattering apparatus:
Slide 6
Procedure
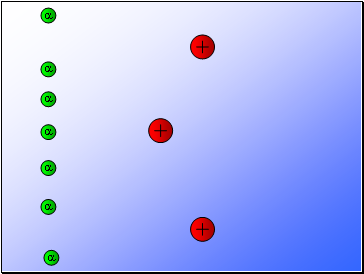
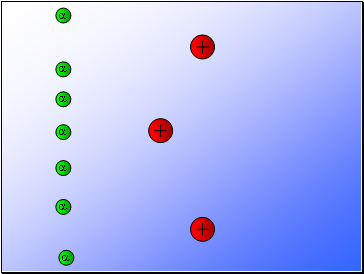
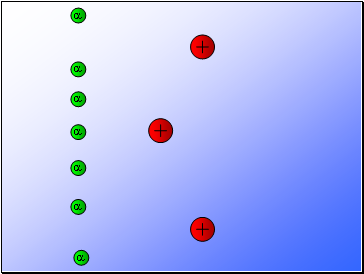
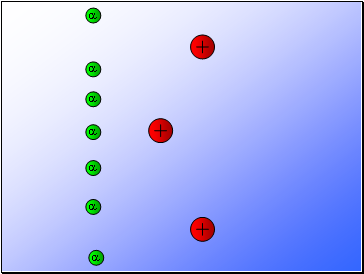
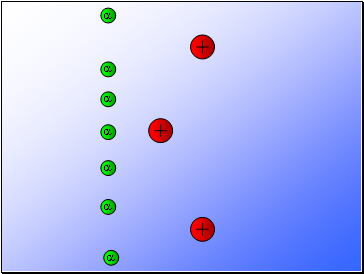
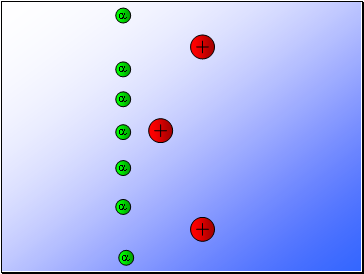
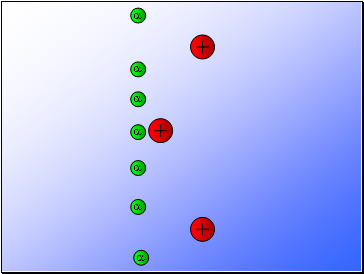
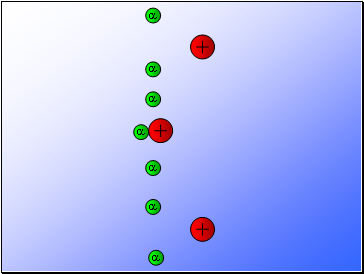
Rutherford fired alpha particles through a piece of gold foil and used a zinc sulphide detector to detect the scattered alpha particles and their location.
Slide 7
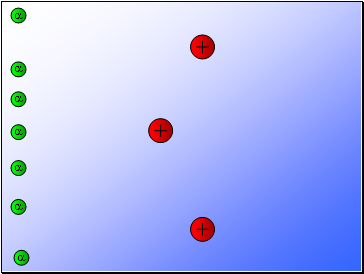
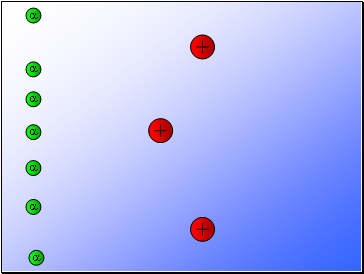
Results…
Slide 8
Slide 9
Slide 10
Slide 11
Slide 12
Slide 13
Slide 14
Slide 15
Slide 16
Slide 17
Slide 18
a
a
a
a
a
a
Slide 19
Contents
Last added presentations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Sensory and Motor Mechanisms
- Mechanics Lecture
- Friction
- Waves & Sound
- Newton’s law of universal gravitation
- Madame Marie Curie