ElectrochemistryPage
2
2
Active nonmetals:
Gain electrons easily
Are easily reduced
Are strong oxidizing agents
Slide 12
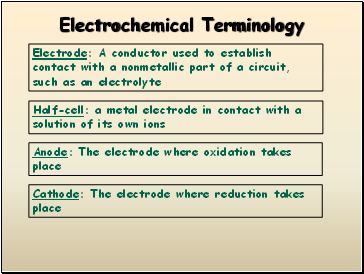
Electrochemical Terminology
Half-cell: a metal electrode in contact with a solution of its own ions
Electrode: A conductor used to establish contact with a nonmetallic part of a circuit, such as an electrolyte
Anode: The electrode where oxidation takes place
Cathode: The electrode where reduction takes place
Slide 13
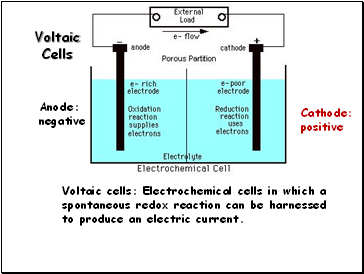
Voltaic Cells
Voltaic cells: Electrochemical cells in which a spontaneous redox reaction can be harnessed to produce an electric current.
Anode:
negative
Cathode:
positive
Slide 14
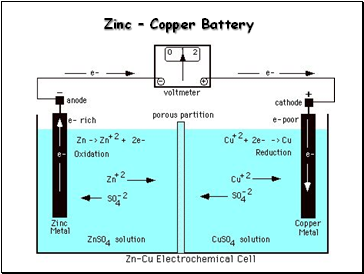
Zinc – Copper Battery
Slide 15
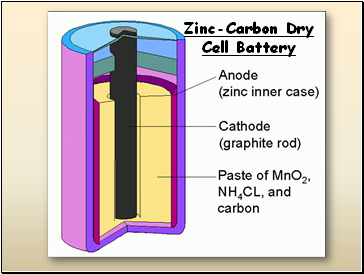
Zinc-Carbon Dry Cell Battery
Slide 16
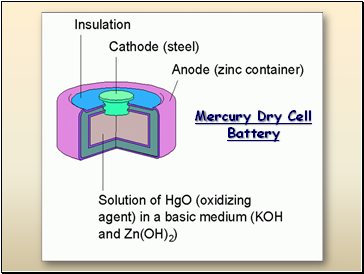
Mercury Dry Cell Battery
Slide 17
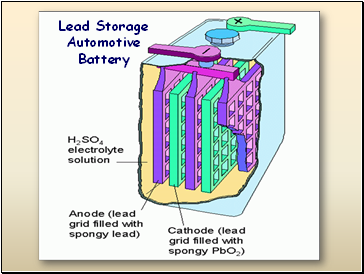
Lead Storage Automotive Battery
Slide 18
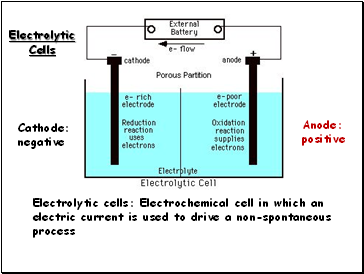
Electrolytic Cells
Electrolytic cells: Electrochemical cell in which an electric current is used to drive a non-spontaneous process
Cathode:
negative
Anode:
positive
Slide 19
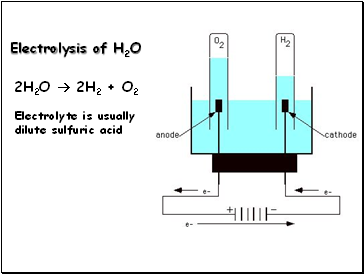
Electrolysis of H2O
2H2O 2H2 + O2
Electrolyte is usually dilute sulfuric acid
Slide 20
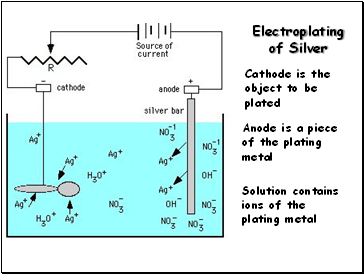
Electroplating of Silver
Cathode is the object to be plated
Anode is a piece of the plating metal
Solution contains ions of the plating metal
Slide 21
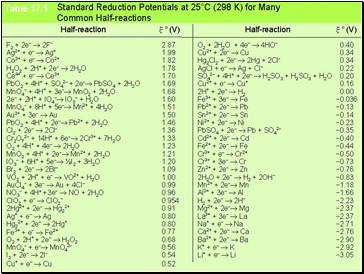
Standard Reduction Potentials
Slide 22
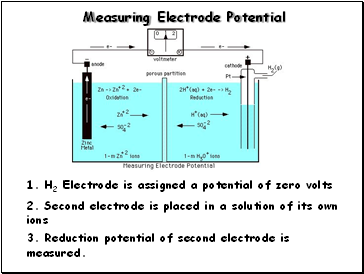
Measuring Electrode Potential
1. H2 Electrode is assigned a potential of zero volts
2. Second electrode is placed in a solution of its own ions
3. Reduction potential of second electrode is measured.
1 2
Contents
- Oxidation and Reduction (Redox)
- Oxidation Reduction Reactions (Redox)
- LEO says GER :
- Not All Reactions are Redox Reactions
- Rules for Assigning Oxidation Numbers
- Reducing Agents and Oxidizing Agents
- Trends in Oxidation and Reduction
- Electrochemical Terminology
- Electrolytic Cells
- Electrolysis of H2O
- Electroplating of Silver
- Measuring Electrode Potential
Last added presentations
- Radiation
- Solar Thermal Energy
- Mechanics Lecture
- Radioactivity and Nuclear Reactions
- Heat-Energy on the Move
- Buoyancy
- Space Radiation