Acids & BasesPage
1
1
Slide 1
Acids & Bases
By Robert McGee
Slide 2
Our Goals for today
To determine the difference between Acids & Bases
Discuss the importance of studying Acids & Bases
Perform an experiment dealing with Acids & Bases
Slide 3
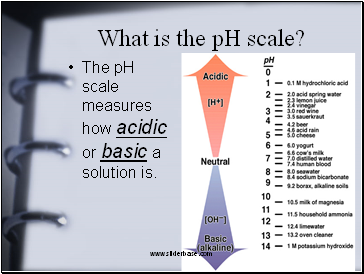
What is the pH scale?
The pH scale measures how acidic or basic a solution is.
Slide 4
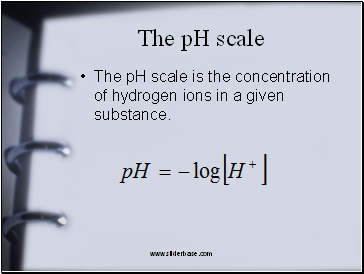
The pH scale
The pH scale is the concentration of hydrogen ions in a given substance.
Slide 5
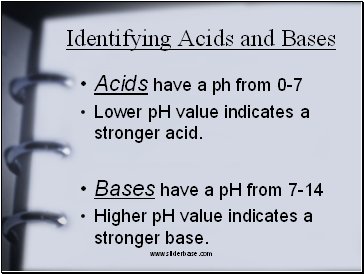
Identifying Acids and Bases
Acids have a ph from 0-7
Lower pH value indicates a stronger acid.
Bases have a pH from 7-14
Higher pH value indicates a stronger base.
Slide 6
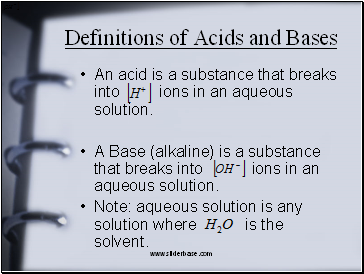
Definitions of Acids and Bases
An acid is a substance that breaks into ions in an aqueous solution.
A Base (alkaline) is a substance that breaks into ions in an aqueous solution.
Note: aqueous solution is any solution where is the solvent.
Slide 7
Did we Miss something??
What happens when the pH of a substance is 7?
Ans: A pH level of 7 indicates a Neutral Substance i.e: Water!
Slide 8
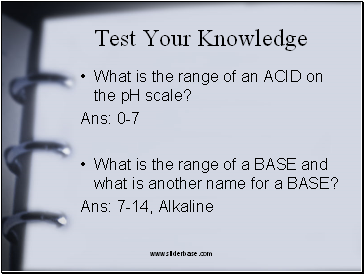
Test Your Knowledge
What is the range of an ACID on the pH scale?
Ans: 0-7
What is the range of a BASE and what is another name for a BASE?
Ans: 7-14, Alkaline
Slide 9
Characteristics Of Acids
Acids can be characterized by:
A sour taste.
It turns blue litmus paper red
It tastes sour. Try drinking lemon juice (citric acid)
Slide 10
Characteristics of Bases
A Base is characterized by:
A bitter taste. (Milk of Magnesia)
It feels slippery. (Soapy Water)
It turns Red Litmus Blue.
Slide 11
Why Learn about Acids & Bases?
What do you think is the pH level of NYC tap water?
The pH of a swimming pool must be checked periodically. Why?
Is it important for Lakes & Rivers to maintain a certain pH?
Slide 12
1 2
Contents
- Our Goals for today
- What is the pH scale?
- The pH scale
- Identifying Acids and Bases
- Definitions of Acids and Bases
- Did we Miss something??
- Characteristics Of Acids
- Characteristics of Bases
- Why Learn about Acids & Bases?
Last added presentations
- Practical Applications of Solar Energy
- Motion
- The Effects of Radiation on Living Things
- Heat-Energy on the Move
- Upcoming Classes
- Thermal Energy
- Ch 9 Nuclear Radiation