Balancing Chemical Equations. Balancing redox equationsPage
2
2
(b) NO2(g) + H2(g) NH3(g) + H2O(g)
Answer: NO2(g) + 3 ½ H2(g) NH3(g) + 2H2O(g)
(c) SO2(g) + O2(g) SO3(g)
Answer: 2SO2(g) + O2(g) 2SO3(g)
Slide 6
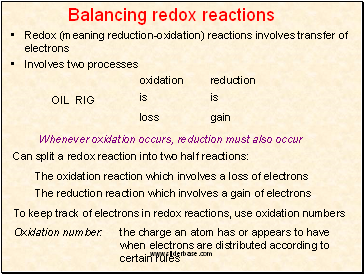
Balancing redox reactions
Redox (meaning reduction-oxidation) reactions involves transfer of electrons
Involves two processes
oxidation
is
loss
reduction
is
gain
Can split a redox reaction into two half reactions:
The oxidation reaction which involves a loss of electrons
The reduction reaction which involves a gain of electrons
To keep track of electrons in redox reactions, use oxidation numbers
Oxidation number: the charge an atom has or appears to have when electrons are distributed according to certain rules
OIL RIG
Whenever oxidation occurs, reduction must also occur
Slide 7
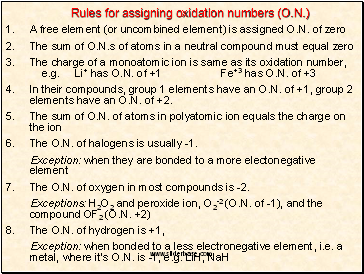
Rules for assigning oxidation numbers (O.N.)
A free element (or uncombined element) is assigned O.N. of zero
The sum of O.N.s of atoms in a neutral compound must equal zero
The charge of a monoatomic ion is same as its oxidation number, e.g. Li+ has O.N. of +1 Fe+3 has O.N. of +3
In their compounds, group 1 elements have an O.N. of +1, group 2 elements have an O.N. of +2.
The sum of O.N. of atoms in polyatomic ion equals the charge on the ion
The O.N. of halogens is usually -1.
Exception: when they are bonded to a more electonegative element
7. The O.N. of oxygen in most compounds is -2.
Exceptions: H2O2 and peroxide ion, O2-2 (O.N. of -1), and the compound OF2 (O.N. +2)
8. The O.N. of hydrogen is +1,
Exception: when bonded to a less electronegative element, i.e. a metal, where itís O.N. is -1, e.g. LiH, NaH
Slide 8
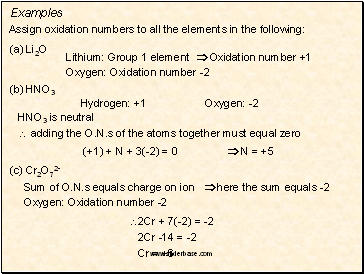
Examples
Assign oxidation numbers to all the elements in the following:
(a) Li2O
Lithium: Group 1 element Oxidation number +1
Oxygen: Oxidation number -2
(b) HNO3
Hydrogen: +1
Oxygen: -2
HNO3 is neutral
adding the O.N.s of the atoms together must equal zero
(+1) + N + 3(-2) = 0 N = +5
(c) Cr2O72-
Sum of O.N.s equals charge on ion here the sum equals -2
Oxygen: Oxidation number -2
2Cr + 7(-2) = -2
2Cr -14 = -2
Cr = +6
Slide 9
Questions
Assign oxidation numbers to each of the following:
(a) ZnCl2
Answer: Zn: +2 Cl: -1
(b) SO3
Answer: O: -2 S: +6
(c) MnO4-
Answer: O: -2 Mn: +7
Slide 10
Defining Oxidising and Reducing agents
Remember that for an oxidation to occur, a reduction must also occur
Contents
- Balancing Chemical Equations
- Balancing redox reactions
- Rules for assigning oxidation numbers (O.N.)
- Defining Oxidising and Reducing agents
- Identifying Oxidising and Reducing Agents
- Balancing redox equations
Last added presentations
- Mechanics Lecture
- Ch 9 Nuclear Radiation
- Gravitation
- Thermal Energy
- Radiation
- Sound
- Radiation Safety and Operations