MineralsPage
2
2
Covalent (O2)
Metallic (Cu, Al, Fe)
Hydrogen (in water)
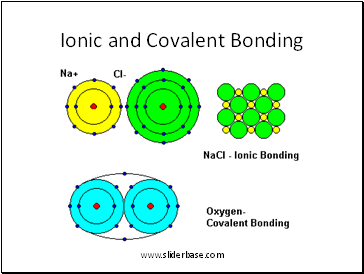
2. Atoms bond by sharing electrons
Slide 13
Ionic and Covalent Bonding
2. Atoms bond by sharing electrons
Slide 14
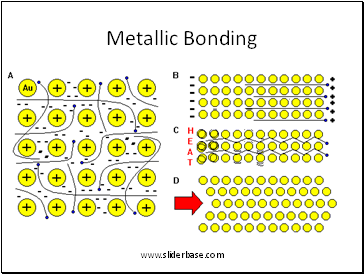
Metallic Bonding
2. Atoms bond by sharing electrons
Slide 15
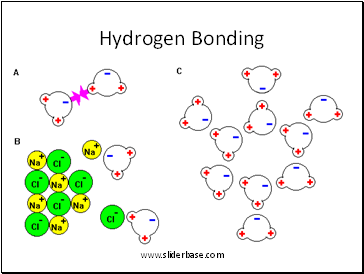
Hydrogen Bonding
2. Atoms bond by sharing electrons
Slide 16
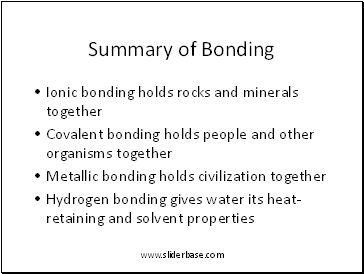
Summary of Bonding
Ionic bonding holds rocks and minerals together
Covalent bonding holds people and other organisms together
Metallic bonding holds civilization together
Hydrogen bonding gives water its heat-retaining and solvent properties
2. Atoms bond by sharing electrons
Slide 17
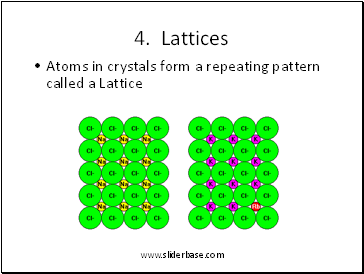
4. Lattices
Atoms in crystals form a repeating pattern called a Lattice
2. Atoms bond by sharing electrons
Slide 18
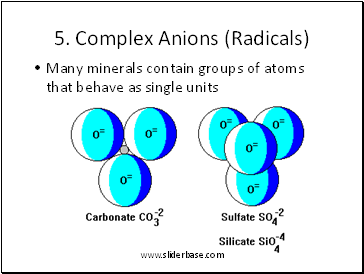
5. Complex Anions (Radicals)
Many minerals contain groups of atoms that behave as single units
2. Atoms bond by sharing electrons
Slide 19
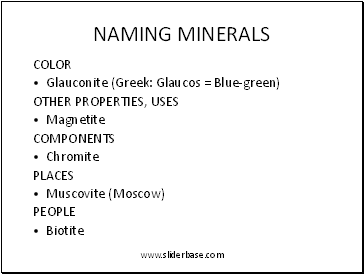
Naming minerals
COLOR
Glauconite (Greek: Glaucos = Blue-green)
OTHER PROPERTIES, USES
Magnetite
COMPONENTS
Chromite
PLACES
Muscovite (Moscow)
PEOPLE
Biotite
Slide 20
Chemicals (and Minerals) Are Classified by their Anions
3. Minerals are classified by their chemistry
Slide 21
For Example: Iron Compounds Have Little in Common
Fe: Gray, Metallic
FeCl2: Light Green, Water Soluble
FeSO4: Light Green, Water Soluble
FeCO3: Brown, Fizzes in Acid
FeS2: Dense, Brittle, Metallic, Cubic Crystals
3. Minerals are classified by their chemistry
Slide 22
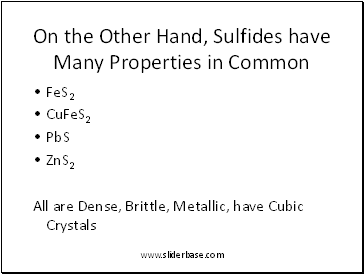
On the Other Hand, Sulfides have Many Properties in Common
FeS2
CuFeS2
PbS
ZnS2
All are Dense, Brittle, Metallic, have Cubic Crystals
3. Minerals are classified by their chemistry
Slide 23
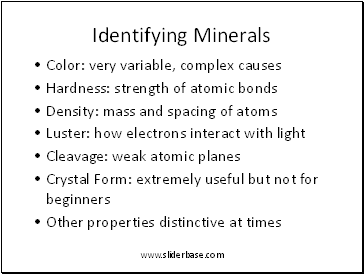
Identifying Minerals
4. Minerals can be identified by their physical properties
Slide 24
Identifying Minerals
Contents
- Take-Away Points
- Composition of the Sun
- How Elements Form in Stars
- What are Planets Made of?
- Minerals are the Chemicals that make up the Earth
- Summary of Bonding
- Naming minerals
- Identifying Minerals
- Color
- Hardness
- Density
- Luster
- Cleavage
- Identifying Minerals
- Unit Cells
Last added presentations
- Space Radiation
- Newton’s laws of motion
- Sound
- Solar Thermal Energy
- Ch 9 Nuclear Radiation
- Static and Kinetic Friction
- Motion