MineralsPage
3
3
Color: very variable, complex causes
Hardness: strength of atomic bonds
Density: mass and spacing of atoms
Luster: how electrons interact with light
Cleavage: weak atomic planes
Crystal Form: extremely useful but not for beginners
Other properties distinctive at times
4. Minerals can be identified by their physical properties = atomic structure
Slide 25
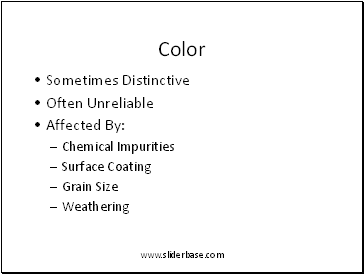
Color
Sometimes Distinctive
Often Unreliable
Affected By:
Chemical Impurities
Surface Coating
Grain Size
Weathering
4. Minerals can be identified by their physical properties = atomic structure
Slide 26
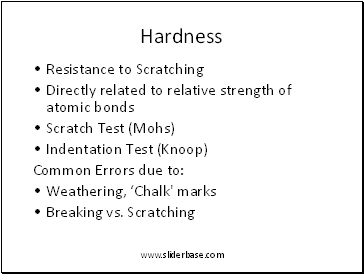
Hardness
Resistance to Scratching
Directly related to relative strength of atomic bonds
Scratch Test (Mohs)
Indentation Test (Knoop)
Common Errors due to:
Weathering, ‘Chalk' marks
Breaking vs. Scratching
4. Minerals can be identified by their physical properties = atomic structure
Slide 27
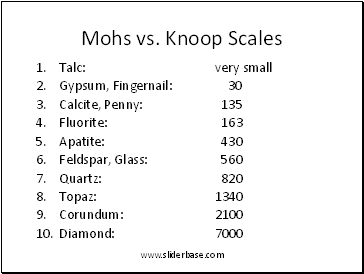
Mohs vs. Knoop Scales
Talc: very small
Gypsum, Fingernail: 30
Calcite, Penny: 135
Fluorite: 163
Apatite: 430
Feldspar, Glass: 560
Quartz: 820
Topaz: 1340
Corundum: 2100
Diamond: 7000
4. Minerals can be identified by their physical properties = atomic structure
Slide 28
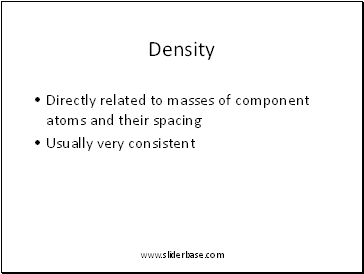
Density
Directly related to masses of component atoms and their spacing
Usually very consistent
4. Minerals can be identified by their physical properties = atomic structure
Slide 29
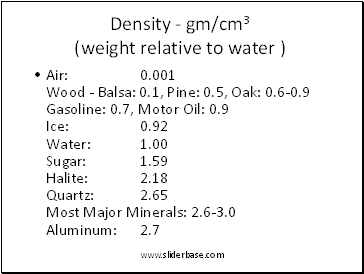
Density - gm/cm3 (weight relative to water )
Air: 0.001 Wood - Balsa: 0.1, Pine: 0.5, Oak: 0.6-0.9 Gasoline: 0.7, Motor Oil: 0.9 Ice: 0.92 Water: 1.00 Sugar: 1.59 Halite: 2.18 Quartz: 2.65 Most Major Minerals: 2.6-3.0 Aluminum: 2.7
4. Minerals can be identified by their physical properties = atomic structure
Slide 30
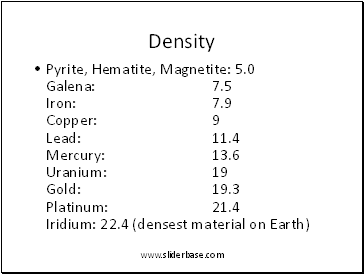
Density
Pyrite, Hematite, Magnetite: 5.0 Galena: 7.5 Iron: 7.9 Copper: 9 Lead: 11.4 Mercury: 13.6 Uranium: 19 Gold: 19.3 Platinum: 21.4 Iridium: 22.4 (densest material on Earth)
4. Minerals can be identified by their physical properties = atomic structure
Slide 31
Luster
Metallic or Nonmetallic is the most important distinction.
Resinous, waxy, silky, etc. are self-explanatory.
Vitreous is often used for glassy luster.
4. Minerals can be identified by their physical properties = atomic structure
Contents
- Take-Away Points
- Composition of the Sun
- How Elements Form in Stars
- What are Planets Made of?
- Minerals are the Chemicals that make up the Earth
- Summary of Bonding
- Naming minerals
- Identifying Minerals
- Color
- Hardness
- Density
- Luster
- Cleavage
- Identifying Minerals
- Unit Cells
Last added presentations
- Newton's Laws
- Radiation
- Simulation at NASA for the Space Radiation Effort
- Ch 9 Nuclear Radiation
- Motion
- Radiation Safety and Operations
- Waves & Sound