Polar Covalent Bonds Acids and BasesPage
6
6
molecules
Slide 39
39
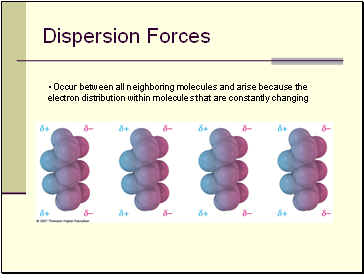
Dispersion Forces
• Occur between all neighboring molecules and arise because the electron distribution within molecules that are constantly changing
Slide 40
40
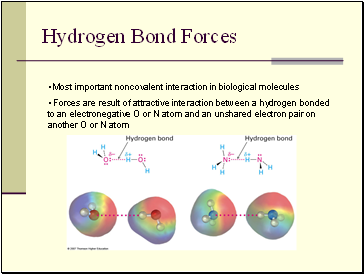
Hydrogen Bond Forces
• Most important noncovalent interaction in biological molecules
• Forces are result of attractive interaction between a hydrogen bonded to an electronegative O or N atom and an unshared electron pair on another O or N atom
Slide 41
41
Slide 42
42
Summary
Organic molecules often have polar covalent bonds as a result of unsymmetrical electron sharing caused by differences in the electronegativity of atoms
The polarity of a molecule is measured by its dipole moment, .
(+) and () indicate formal charges on atoms in molecules to keep track of valence electrons around an atom
Some substances must be shown as a resonance hybrid of two or more resonance forms that differ by the location of electrons.
A Brønsted(–Lowry) acid donates a proton
A Brønsted(–Lowry) base accepts a proton
The strength Brønsted acid is related to the -1 times the logarith of the acidity constant, pKa. Weaker acids have higher pKa’s
Slide 43
43
Summary (cont’d)
A Lewis acid has an empty orbital that can accept an electron pair
A Lewis base can donate an unshared electron pair
In condensed structures C-C and C-H are implied
Skeletal structures show bonds and not C or H (C is shown as a junction of two lines) – other atoms are shown
Molecular models are useful for representing structures for study
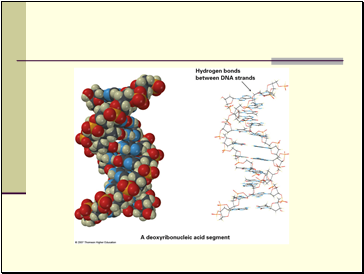
Noncovalent interactions have several types: dipole-dipole, dispersion, and hydrogen bond forces
Contents
- Why this chapter?
- Polar Covalent Bonds: Electronegativity
- Bond Polarity and Electronegativity
- Electrostatic Potential Maps
- Polar Covalent Bonds: Dipole Moments
- Formal Charges
- Resonance
- Rules for Resonance Forms
- Drawing Resonance Forms
- Pentanedione
- Acids and Bases: The Brønsted–Lowry Definition
- Acid and Base Strength
- Predicting Acid–Base Reactions from pKa Values
- Organic Acids and Organic Bases
- Acids and Bases: The Lewis Definition
- Molecular Models
- Noncovalent Interactions
Last added presentations
- Radioactivity and Nuclear Reactions
- The Effects of Radiation on Living Things
- Practical Applications of Solar Energy
- Sound
- Newton’s Law of Gravity
- Geophysical Concepts, Applications and Limitations
- Newton’s Laws of Motion