Quantum Theory of the AtomPage
7
7
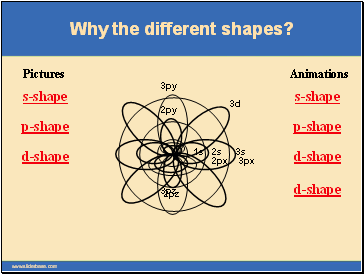
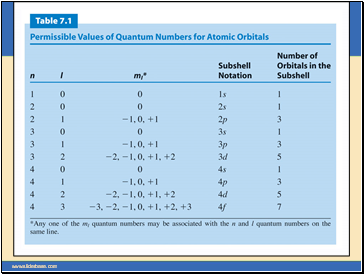
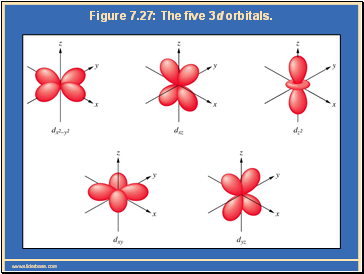
Each main “shell” is subdivided into “sub shells.” Within each shell of quantum number n, there are n sub shells, each with a distinctive shape.
l can have any integer value from 0 to (n - 1)
The different subshells are denoted by letters.
Letter s p d f g …
l 0 1 2 3 4 ….
Quantum Numbers and Atomic Orbitals
Slide 41
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–41
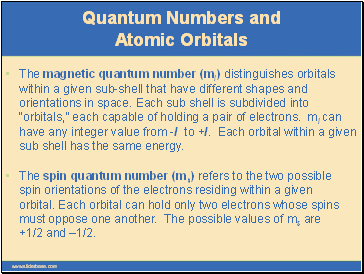
The magnetic quantum number (ml) distinguishes orbitals within a given sub-shell that have different shapes and orientations in space. Each sub shell is subdivided into “orbitals,” each capable of holding a pair of electrons. ml can have any integer value from -l to +l. Each orbital within a given sub shell has the same energy.
Quantum Numbers and Atomic Orbitals
The spin quantum number (ms) refers to the two possible spin orientations of the electrons residing within a given orbital. Each orbital can hold only two electrons whose spins must oppose one another. The possible values of ms are +1/2 and –1/2.
Slide 42
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–42
Why the different shapes?
1s
3s
2s
2px
3pz
3d
2py
2pz
3py
3px
s-shape
p-shape
d-shape
d-shape
Animations
Pictures
s-shape
p-shape
d-shape
Slide 43
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–43
Scanning Tunneling Microscope Electron Clouds
Image Movie
Slide 44
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–44
Slide 45
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–45
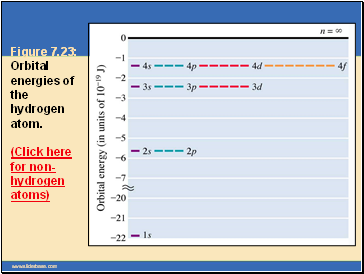
Figure 7.23: Orbital energies of the hydrogen atom. (Click here for non-hydrogen atoms)
Slide 46
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–46
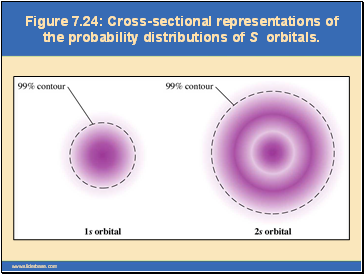
Figure 7.24: Cross-sectional representations of the probability distributions of S orbitals.
Slide 47
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–47
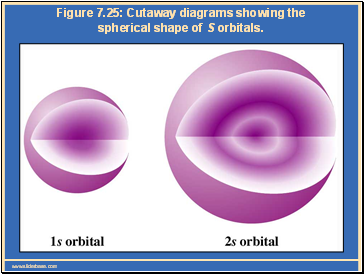
Figure 7.25: Cutaway diagrams showing the spherical shape of S orbitals.
Slide 48
Contents
- The Wave Nature of Light
- What is Quantized?
- Quantum Effects and Photons
- Radio Wave Energy
- A Problem to Consider
Last added presentations
- Newton’s third law of motion
- Sensory and Motor Mechanisms
- Heat-Energy on the Move
- Health Physics
- Mechanics Lecture
- Thermal Energy
- Magnetic field uses sound waves to ignite sun's ring of fire