Quantum Theory of the AtomPage
4
4
Atomic Line Spectra
In 1885, J. J. Balmer showed that the wavelengths, l, in the visible spectrum of hydrogen could be reproduced by a simple formula.
The known wavelengths of the four visible lines for hydrogen correspond to values of n = 3, n = 4, n = 5, and n = 6.
The Bohr Theory of the Hydrogen Atom
Slide 22
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–22
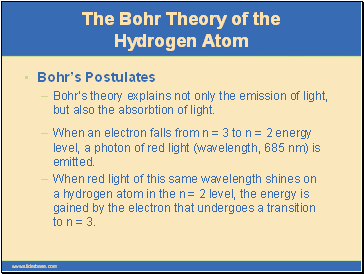
Bohr’s Postulates
Bohr’s theory explains not only the emission of light, but also the absorbtion of light.
When an electron falls from n = 3 to n = 2 energy level, a photon of red light (wavelength, 685 nm) is emitted.
When red light of this same wavelength shines on a hydrogen atom in the n = 2 level, the energy is gained by the electron that undergoes a transition to n = 3.
The Bohr Theory of the Hydrogen Atom
Slide 23
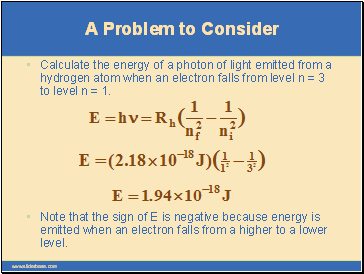
A Problem to Consider
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–23
Calculate the energy of a photon of light emitted from a hydrogen atom when an electron falls from level n = 3 to level n = 1.
Note that the sign of E is negative because energy is emitted when an electron falls from a higher to a lower level.
Slide 24
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–24
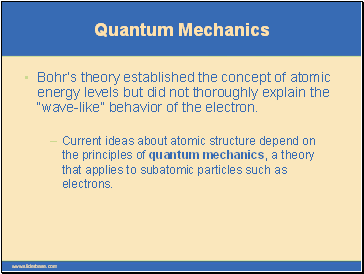
Quantum Mechanics
Bohr’s theory established the concept of atomic energy levels but did not thoroughly explain the “wave-like” behavior of the electron.
Current ideas about atomic structure depend on the principles of quantum mechanics, a theory that applies to subatomic particles such as electrons.
Slide 25
Quantum Theory of the Atom
7.4 Quantum Mechanics 7.5 Quantum Numbers and Atomic Orbitals
Slide 26
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–26
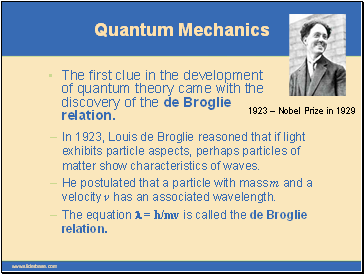
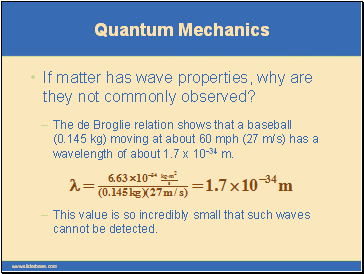
The first clue in the development of quantum theory came with the discovery of the de Broglie relation.
In 1923, Louis de Broglie reasoned that if light exhibits particle aspects, perhaps particles of matter show characteristics of waves.
He postulated that a particle with mass m and a velocity v has an associated wavelength.
The equation l = h/mv is called the de Broglie relation.
Quantum Mechanics
1923 – Nobel Prize in 1929
Slide 27
.Copyright © Houghton Mifflin Company.All rights reserved.
Contents
- The Wave Nature of Light
- What is Quantized?
- Quantum Effects and Photons
- Radio Wave Energy
- A Problem to Consider
Last added presentations
- Heat-Energy on the Move
- Motion
- Simulation at NASA for the Space Radiation Effort
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Mechanics Lecture
- Newton's Laws
- Buoyancy