Quantum Theory of the AtomPage
5
5
Presentation of Lecture Outlines, 7–27
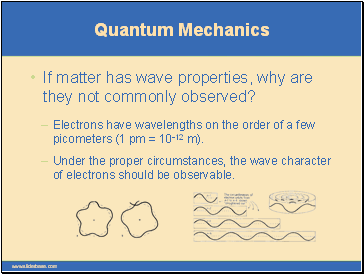
If matter has wave properties, why are they not commonly observed?
The de Broglie relation shows that a baseball (0.145 kg) moving at about 60 mph (27 m/s) has a wavelength of about 1.7 x 10-34 m.
This value is so incredibly small that such waves cannot be detected.
Quantum Mechanics
Slide 28
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–28
Electrons have wavelengths on the order of a few picometers (1 pm = 10-12 m).
Under the proper circumstances, the wave character of electrons should be observable.
Quantum Mechanics
If matter has wave properties, why are they not commonly observed?
Slide 29
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–29
Quantum Mechanics
In 1927, it was demonstrated that a beam of electrons, just like X rays, could be diffracted by a crystal.
The German physicist, Ernst Ruska, used this wave property to construct the first “electron microscope” in 1933.
Slide 30
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–30
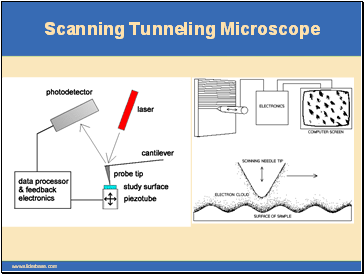
Scanning Tunneling Microscope
Slide 31
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–31
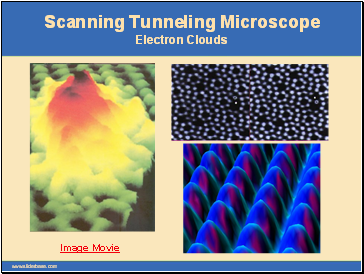
Scanning Tunneling Microscope Electron Clouds
Image Movie
Slide 32
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–32
Quantum Mechanics
Quantum mechanics is the branch of physics that mathematically describes the wave properties of submicroscopic particles.
We can no longer think of an electron as having a precise orbit in an atom.
To describe such an orbit would require knowing its exact position and velocity.
In 1927, Werner Heisenberg showed (from quantum mechanics) that it is impossible to know both simultaneously.
Slide 33
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–33
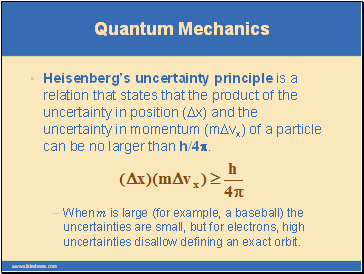
Quantum Mechanics
Heisenberg’s uncertainty principle is a relation that states that the product of the uncertainty in position (Dx) and the uncertainty in momentum (mDvx) of a particle can be no larger than h/4p.
When m is large (for example, a baseball) the uncertainties are small, but for electrons, high uncertainties disallow defining an exact orbit.
Contents
- The Wave Nature of Light
- What is Quantized?
- Quantum Effects and Photons
- Radio Wave Energy
- A Problem to Consider
Last added presentations
- Waves & Sound
- Resource Acquisition and Transport in Vascular Plants
- Radiation
- The Effects of Radiation on Living Things
- Soil and Plant Nutrition
- Sound
- Practical Applications of Solar Energy