Quantum Theory of the AtomPage
2
2
Maxwell Planck-Black Body Radiation
Found that blackbody radiation was quantized.
1900—Nobel Prize in 1918
Slide 9
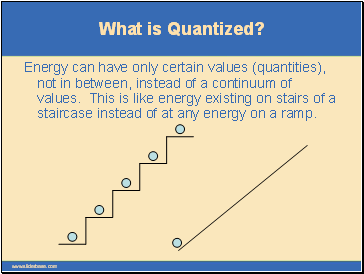
What is Quantized?
Copyright © Houghton Mifflin Company.All rights reserved.
Energy can have only certain values (quantities), not in between, instead of a continuum of values. This is like energy existing on stairs of a staircase instead of at any energy on a ramp.
Slide 10
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–10
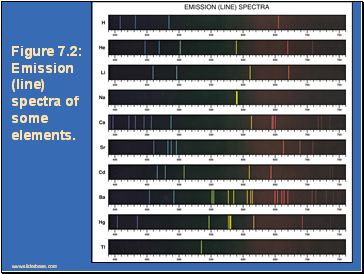
Figure 7.2: Emission (line) spectra of some elements.
Slide 11
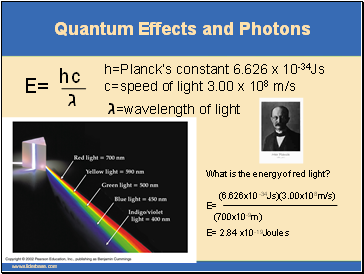
Quantum Effects and Photons
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–11
E=
ג
hc
h=Planck’s constant 6.626 x 10-34Js c=speed of light 3.00 x 108 m/s ג=wavelength of light
What is the energy of red light? (6.626x10-34Js)(3.00x108m/s) E= (700x10-9m)
E= 2.84 x10-19Joules
Slide 12
Copyright © Houghton Mifflin Company.All rights reserved.
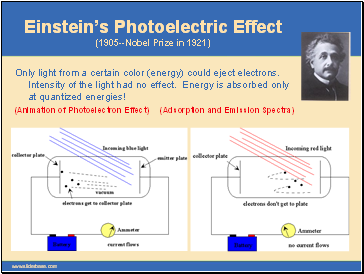
Einstein’s Photoelectric Effect (1905--Nobel Prize in 1921)
Only light from a certain color (energy) could eject electrons. Intensity of the light had no effect. Energy is absorbed only at quantized energies!
(Animation of Photoelectron Effect) (Adsorption and Emission Spectra)
Slide 13
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–13
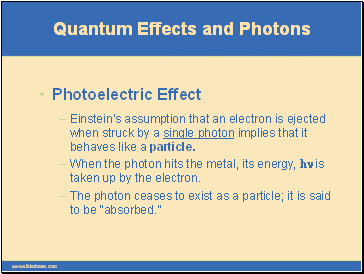
Einstein’s assumption that an electron is ejected when struck by a single photon implies that it behaves like a particle.
Quantum Effects and Photons
Photoelectric Effect
When the photon hits the metal, its energy, hn is taken up by the electron.
The photon ceases to exist as a particle; it is said to be “absorbed.”
Slide 14
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–14
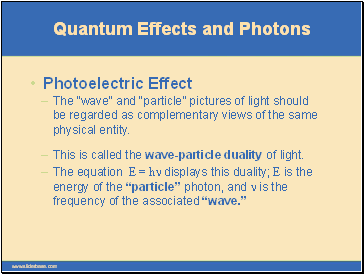
The “wave” and “particle” pictures of light should be regarded as complementary views of the same physical entity.
Quantum Effects and Photons
Photoelectric Effect
This is called the wave-particle duality of light.
The equation E = hn displays this duality; E is the energy of the “particle” photon, and n is the frequency of the associated “wave.”
Slide 15
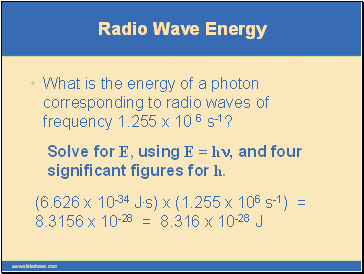
Radio Wave Energy
.Copyright © Houghton Mifflin Company.All rights reserved.
Contents
- The Wave Nature of Light
- What is Quantized?
- Quantum Effects and Photons
- Radio Wave Energy
- A Problem to Consider
Last added presentations
- Gravitation
- Ch 9 Nuclear Radiation
- Newton’s third law of motion
- Newton’s laws of motion
- Newton's laws of motion
- Soil and Plant Nutrition
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal