Structure of the AtomPage
2
2
If an α particle were scattered by many electrons and N electrons results in .
The number of atoms across the thin gold layer of 6 × 10−7 m:
Assume the distance between atoms is
and there are .
That gives .
Slide 8
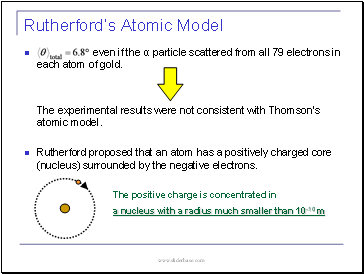
Rutherford’s Atomic Model
even if the α particle scattered from all 79 electrons in each atom of gold.
The experimental results were not consistent with Thomson’s atomic model.
Rutherford proposed that an atom has a positively charged core (nucleus) surrounded by the negative electrons.
The positive charge is concentrated in
a nucleus with a radius much smaller than 10-10 m
Slide 9
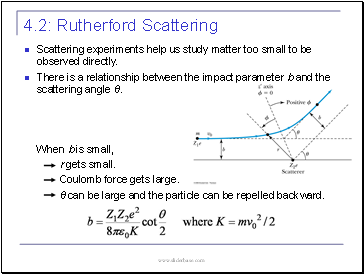
Rutherford Scattering
Scattering experiments help us study matter too small to be observed directly.
There is a relationship between the impact parameter b and the scattering angle θ.
When b is small,
r gets small.
Coulomb force gets large.
θ can be large and the particle can be repelled backward.
4.2:
Slide 10
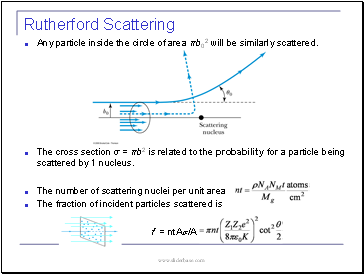
Any particle inside the circle of area πb02 will be similarly scattered.
The cross section σ = πb2 is related to the probability for a particle being scattered by 1 nucleus.
The number of scattering nuclei per unit area .
The fraction of incident particles scattered is
f = ntAs/A
Rutherford Scattering
Slide 11
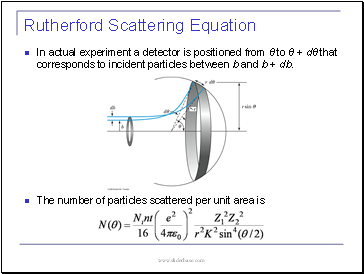
In actual experiment a detector is positioned from θ to θ + dθ that corresponds to incident particles between b and b + db.
The number of particles scattered per unit area is
Rutherford Scattering Equation
Slide 12
The Classical Atomic Model
4.3:
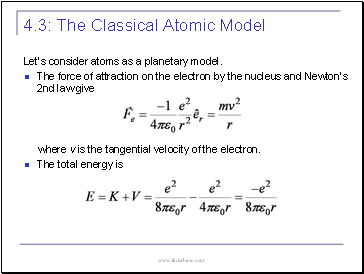
Let’s consider atoms as a planetary model.
The force of attraction on the electron by the nucleus and Newton’s 2nd law give
where v is the tangential velocity of the electron.
The total energy is
Slide 13
The Classical Atomic Model
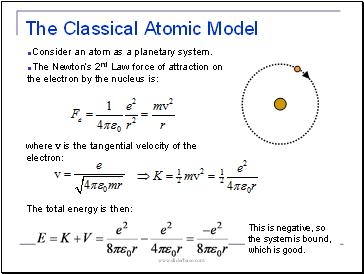
Consider an atom as a planetary system.
The Newton’s 2nd Law force of attraction on the electron by the nucleus is:
where v is the tangential velocity of the electron:
The total energy is then:
This is negative, so the system is bound, which is good.
Slide 14
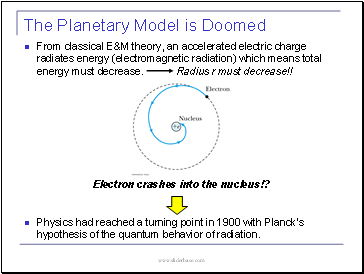
The Planetary Model is Doomed
From classical E&M theory, an accelerated electric charge radiates energy (electromagnetic radiation) which means total energy must decrease. Radius r must decrease!!
Contents
- Structure of the Atom
- Thomson’s Atomic Model
- Radius of an Atom
- Experiments of Geiger and Marsden
- Scattering from 1 electron:
- Multiple Scattering from Electrons
- Rutherford’s Atomic Model
- Rutherford Scattering
- The Classical Atomic Model
- The Bohr Model of the Hydrogen Atom
- Atomic Excitation by Electrons
Last added presentations
- Sound
- Newton's Laws
- Solar Thermal Energy
- Geophysical Concepts, Applications and Limitations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Space Radiation
- Radioactivity and Nuclear Reactions