Structure of the AtomPage
4
4
The ratio of v1 to c is the fine structure constant.
Slide 22
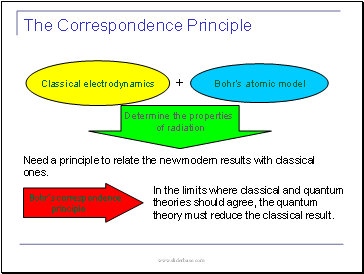
The Correspondence Principle
Need a principle to relate the new modern results with classical ones.
Classical electrodynamics
Bohr’s atomic model
Determine the properties
of radiation
Bohr’s correspondence
principle
In the limits where classical and quantum theories should agree, the quantum theory must reduce the classical result.
+
Slide 23
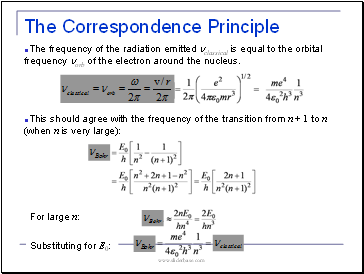
The Correspondence Principle
The frequency of the radiation emitted nclassical is equal to the orbital frequency norb of the electron around the nucleus.
This should agree with the frequency of the transition from n + 1 to n (when n is very large):
For large n:
Substituting for E0:
Slide 24
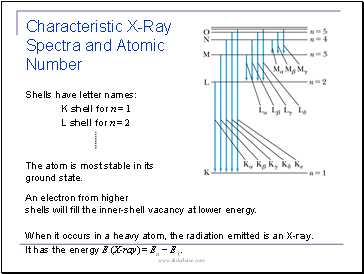
Shells have letter names:
K shell for n = 1
L shell for n = 2
The atom is most stable in its ground state.
When it occurs in a heavy atom, the radiation emitted is an X-ray.
It has the energy E (X-ray) = Eu − Eℓ.
Characteristic X-Ray Spectra and Atomic Number
An electron from higher shells will fill the inner-shell vacancy at lower energy.
Slide 25
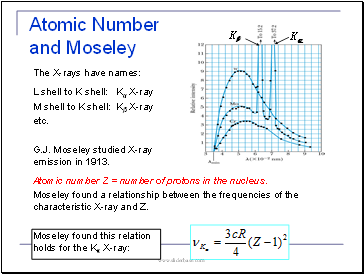
Atomic Number and Moseley
The X-rays have names:
L shell to K shell: Kα X-ray
M shell to K shell: Kβ X-ray
etc.
G.J. Moseley studied X-ray emission in 1913.
Atomic number Z = number of protons in the nucleus.
Moseley found a relationship between the frequencies of the characteristic X-ray and Z.
Moseley found this relation holds for the Kα X-ray:
Slide 26
Moseley’s Empirical Results
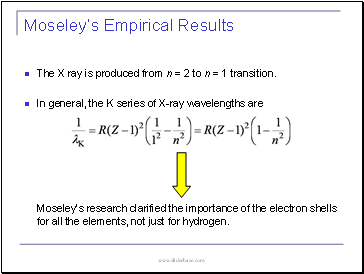
The X ray is produced from n = 2 to n = 1 transition.
In general, the K series of X-ray wavelengths are
Moseley’s research clarified the importance of the electron shells for all the elements, not just for hydrogen.
Slide 27
Atomic Excitation by Electrons
4.7:
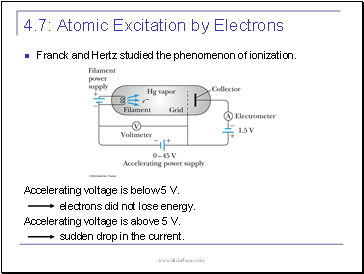
Franck and Hertz studied the phenomenon of ionization.
Accelerating voltage is below 5 V.
electrons did not lose energy.
Accelerating voltage is above 5 V.
sudden drop in the current.
Slide 28
Atomic Excitation by Electrons
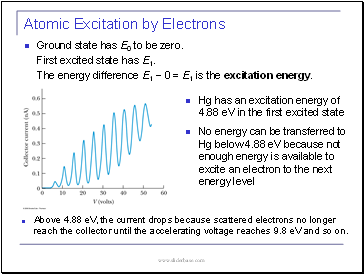
Ground state has E0 to be zero.
First excited state has E1.
The energy difference E1 − 0 = E1 is the excitation energy.
Hg has an excitation energy of 4.88 eV in the first excited state
Contents
- Structure of the Atom
- Thomson’s Atomic Model
- Radius of an Atom
- Experiments of Geiger and Marsden
- Scattering from 1 electron:
- Multiple Scattering from Electrons
- Rutherford’s Atomic Model
- Rutherford Scattering
- The Classical Atomic Model
- The Bohr Model of the Hydrogen Atom
- Atomic Excitation by Electrons
Last added presentations
- Newton’s laws of motion
- Radiation
- Heat-Energy on the Move
- Health Physics
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Magnetic field uses sound waves to ignite sun's ring of fire