Ionic Compound NomenclaturePage
2
2
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced!
( )
2
Slide 14
Writing Ionic Compound Formulas
Example: Ammonium sulfate
1. Write the formulas for the cation and anion, including CHARGES!
NH4+
SO42-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced!
( )
2
Slide 15
Writing Ionic Compound Formulas
Example: Iron(III) chloride
1. Write the formulas for the cation and anion, including CHARGES!
Fe3+
Cl-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced!
3
Slide 16
Writing Ionic Compound Formulas
Example: Aluminum sulfide
1. Write the formulas for the cation and anion, including CHARGES!
Al3+
S2-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced!
2
3
Slide 17
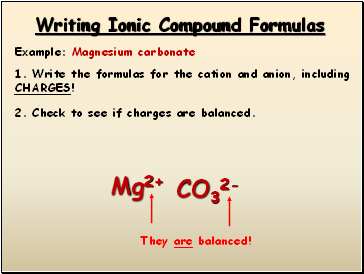
Writing Ionic Compound Formulas
Example: Magnesium carbonate
1. Write the formulas for the cation and anion, including CHARGES!
Mg2+
CO32-
2. Check to see if charges are balanced.
They are balanced!
Slide 18
Writing Ionic Compound Formulas
Example: Zinc hydroxide
1. Write the formulas for the cation and anion, including CHARGES!
Zn2+
OH-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced!
( )
2
Slide 19
Writing Ionic Compound Formulas
Example: Aluminum phosphate
1. Write the formulas for the cation and anion, including CHARGES!
Al3+
PO43-
2. Check to see if charges are balanced.
They ARE balanced!
Slide 20
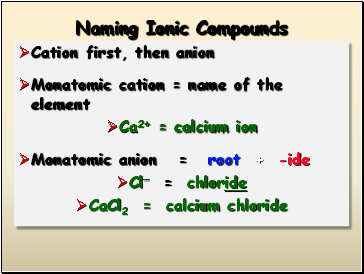
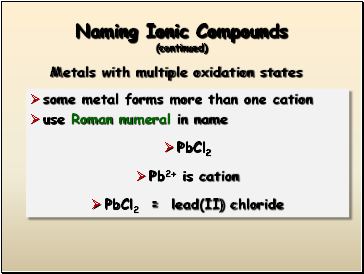
Naming Ionic Compounds
Cation first, then anion
Monatomic cation = name of the element
Ca2+ = calcium ion
Monatomic anion = root + -ide
Cl- = chloride
CaCl2 = calcium chloride
Slide 21
Contents
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Ch 9 Nuclear Radiation
- Sound
- Resource Acquisition and Transport in Vascular Plants
- Health Physics
- Waves & Sound
- Space Radiation