Molecules and IonsPage
3
3
Not balanced
( )
2
Slide 21
Writing Ionic Compound Formulas
Example: Aluminum phosphate
1. Write the formulas for the cation and anion, including CHARGES!
Al3+
PO43-
2. Check to see if charges are balanced.
They ARE balanced
Slide 22
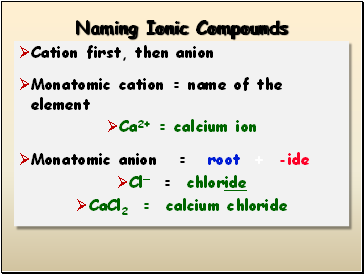
Naming Ionic Compounds
Cation first, then anion
Monatomic cation = name of the element
Ca2+ = calcium ion
Monatomic anion = root + -ide
Cl- = chloride
CaCl2 = calcium chloride
Slide 23
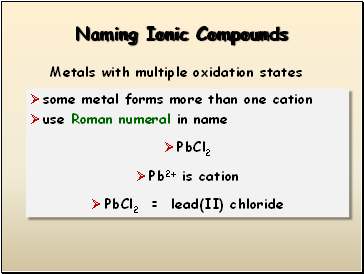
Naming Ionic Compounds
some metal forms more than one cation
use Roman numeral in name
PbCl2
Pb2+ is cation
PbCl2 = lead(II) chloride
Metals with multiple oxidation states
Slide 24
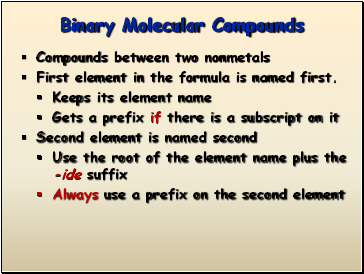
Binary Molecular Compounds
Compounds between two nonmetals
First element in the formula is named first.
Keeps its element name
Gets a prefix if there is a subscript on it
Second element is named second
Use the root of the element name plus the -ide suffix
Always use a prefix on the second element
Slide 25
List of Prefixes
1 = mon(o)
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
10 = deka
Slide 26
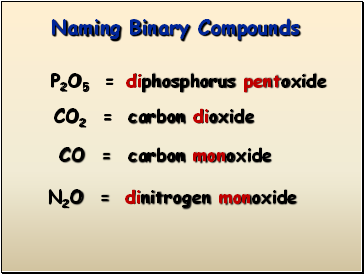
Naming Binary Compounds
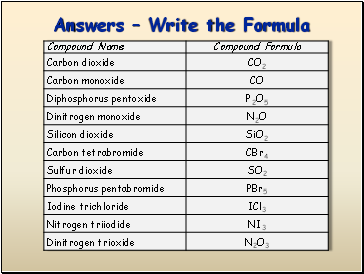
P2O5 =
CO2 =
CO =
N2O =
diphosphorus pentoxide
carbon dioxide
carbon monoxide
dinitrogen monoxide
Slide 27
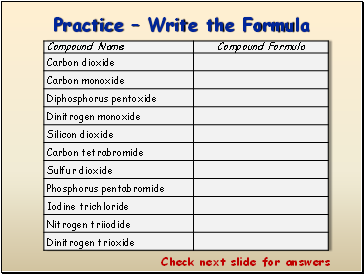
Practice Write the Formula
Check next slide for answers
Slide 28
Answers Write the Formula
Slide 29
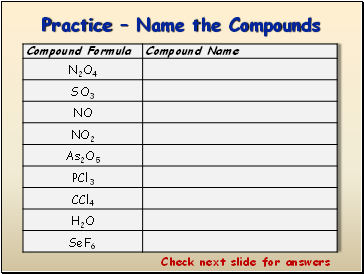
Practice Name the Compounds
Check next slide for answers
Slide 30
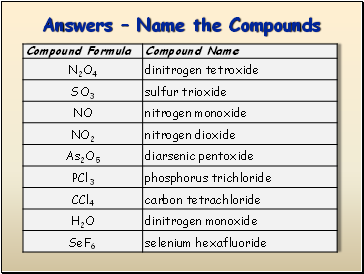
Answers Name the Compounds
Contents
- Molecules
- Covalent Network Substances
- Ions
- Predicting Ionic Charges
- Writing Ionic Compound Formulas
- Naming Ionic Compounds
- Binary Molecular Compounds
- Naming Binary Compounds
- Practice Write the Formula
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Solar Energy
- Static and Kinetic Friction
- Newtons Laws of Motion
- Gravitation
- Waves & Sound
- Buoyancy