Molecules and IonsPage
2
2
Slide 13
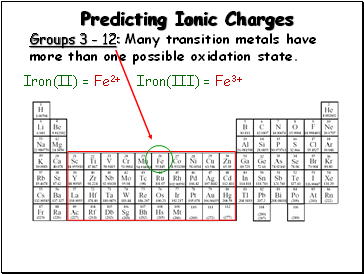
Predicting Ionic Charges
Groups 3 - 12: Many transition metals have more than one possible oxidation state.
Iron(II) = Fe2+
Iron(III) = Fe3+
Slide 14
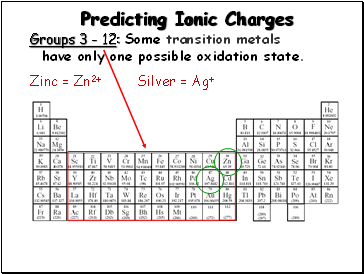
Predicting Ionic Charges
Groups 3 - 12: Some transition metals
have only one possible oxidation state.
Zinc = Zn2+
Silver = Ag+
Slide 15
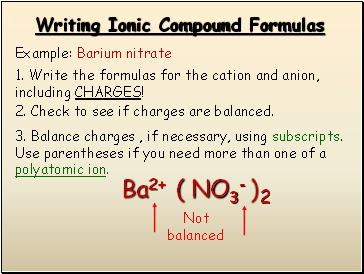
Writing Ionic Compound Formulas
Example: Barium nitrate
1. Write the formulas for the cation and anion, including CHARGES!
Ba2+
NO3-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced
( )
2
Slide 16
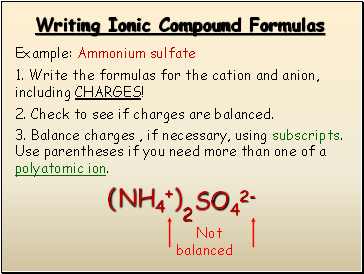
Writing Ionic Compound Formulas
Example: Ammonium sulfate
1. Write the formulas for the cation and anion, including CHARGES!
NH4+
SO42-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced
( )
2
Slide 17
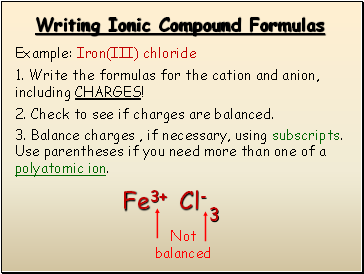
Writing Ionic Compound Formulas
Example: Iron(III) chloride
1. Write the formulas for the cation and anion, including CHARGES!
Fe3+
Cl-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced
3
Slide 18
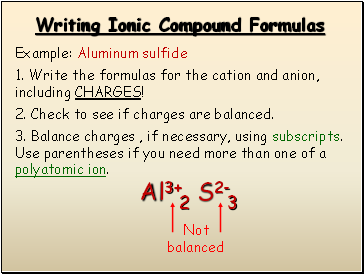
Writing Ionic Compound Formulas
Example: Aluminum sulfide
1. Write the formulas for the cation and anion, including CHARGES!
Al3+
S2-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Not balanced
2
3
Slide 19
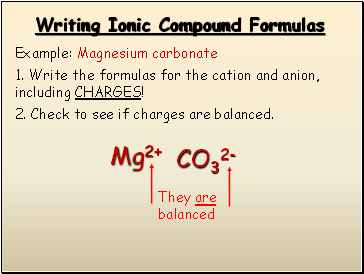
Writing Ionic Compound Formulas
Example: Magnesium carbonate
1. Write the formulas for the cation and anion, including CHARGES!
Mg2+
CO32-
2. Check to see if charges are balanced.
They are balanced
Slide 20
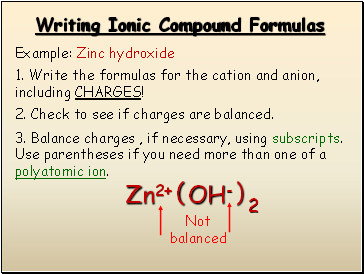
Writing Ionic Compound Formulas
Example: Zinc hydroxide
1. Write the formulas for the cation and anion, including CHARGES!
Zn2+
OH-
2. Check to see if charges are balanced.
3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion.
Contents
- Molecules
- Covalent Network Substances
- Ions
- Predicting Ionic Charges
- Writing Ionic Compound Formulas
- Naming Ionic Compounds
- Binary Molecular Compounds
- Naming Binary Compounds
- Practice – Write the Formula
Last added presentations
- Solar Energy
- Ch 9 Nuclear Radiation
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Heat-Energy on the Move
- Practical Applications of Solar Energy
- Static and Kinetic Friction
- Magnetic field uses sound waves to ignite sun's ring of fire