Molecules and IonsPage
1
1
Slide 1
Molecules and Ions
Image courtesy of www.lab-initio.com
Slide 2
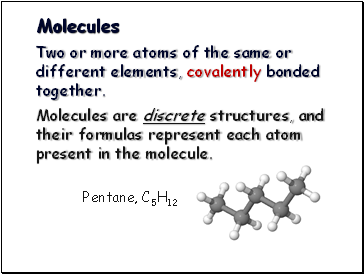
Molecules
Two or more atoms of the same or different elements, covalently bonded together.
Molecules are discrete structures, and their formulas represent each atom present in the molecule.
Pentane, C5H12
Slide 3
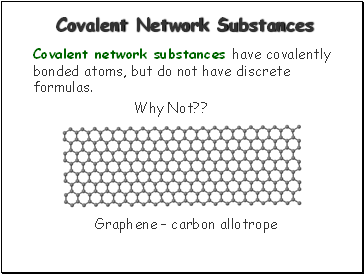
Covalent Network Substances
Covalent network substances have covalently bonded atoms, but do not have discrete formulas.
Why Not??
Graphene – carbon allotrope
Slide 4
Ions
Cation: A positive ion
Mg2+, NH4+
Anion: A negative ion
Cl-, SO42-
Ionic Bonding: Force of attraction between oppositely charged ions.
Ionic compounds form crystals, so their formulas are written empirically (lowest whole number ratio of ions).
Slide 5
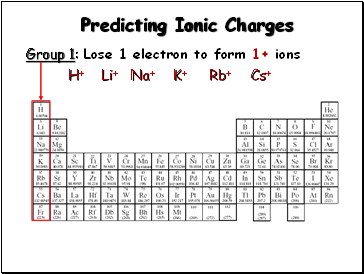
Predicting Ionic Charges
Group 1: Lose 1 electron to form 1+ ions
H+
Li+
Na+
K+
Rb+
Cs+
Slide 6
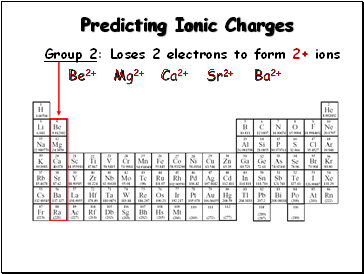
Predicting Ionic Charges
Group 2: Loses 2 electrons to form 2+ ions
Be2+
Mg2+
Ca2+
Sr2+
Ba2+
Slide 7
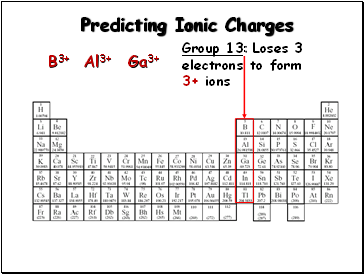
Predicting Ionic Charges
Group 13: Loses 3
electrons to form
3+ ions
B3+
Al3+
Ga3+
Slide 8
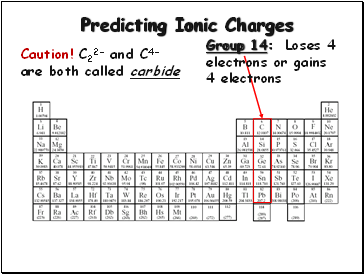
Predicting Ionic Charges
Group 14:
Loses 4
electrons or gains
4 electrons
Caution! C22- and C4- are both called carbide
Slide 9
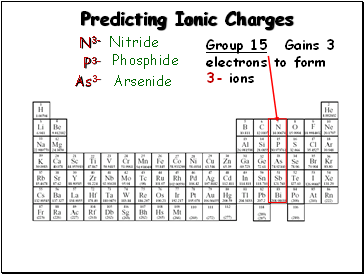
Predicting Ionic Charges
Group 15:
Gains 3
electrons to form
3- ions
N3-
P3-
As3-
Nitride
Phosphide
Arsenide
Slide 10
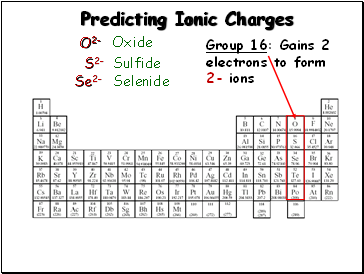
Predicting Ionic Charges
Group 16: Gains 2
electrons to form
2- ions
O2-
S2-
Se2-
Oxide
Sulfide
Selenide
Slide 11
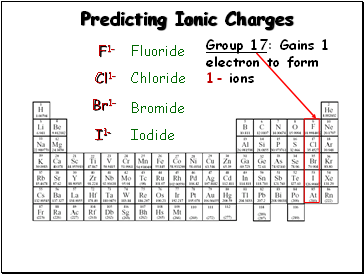
Predicting Ionic Charges
Group 17: Gains 1
electron to form
1- ions
F1-
Cl1-
Br1-
Fluoride
Chloride
Bromide
I1-
Iodide
Slide 12
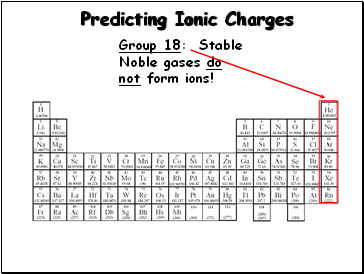
Predicting Ionic Charges
Group 18: Stable Noble gases do not form ions!
Contents
- Molecules
- Covalent Network Substances
- Ions
- Predicting Ionic Charges
- Writing Ionic Compound Formulas
- Naming Ionic Compounds
- Binary Molecular Compounds
- Naming Binary Compounds
- Practice – Write the Formula
Last added presentations
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Direct heat utilization of geothermal energy
- Solar Energy
- Solar Thermal Energy
- The Effects of Radiation on Living Things
- History of Modern Astronomy
- Mechanics Lecture