Water and the Fitness of the EnvironmentPage
5
5
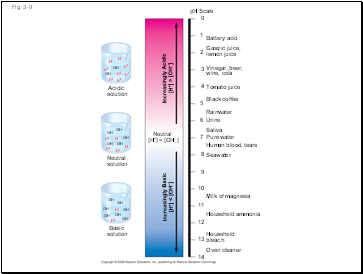
Acidic solutions have pH values less than 7
Basic solutions have pH values greater than 7
Most biological fluids have pH values in the range of 6 to 8
Slide 39
Fig. 3-9
Neutral
solution
Acidic
solution
Basic
solution
OH–
OH–
OH–
OH–
OH–
OH–
OH–
H+
H+
H+
OH–
H+
H+
H+
H+
OH–
OH–
OH–
OH–
H+
OH–
H+
H+
H+
H+
H+
H+
H+
OH–
Neutral
[H+] = [OH–]
Increasingly Acidic
[H+] > [OH–]
Increasingly Basic
[H+] < [OH–]
pH Scale
0
1
2
3
4
5
6
7
8
Battery acid
Gastric juice,
lemon juice
Vinegar, beer,
wine, cola
Tomato juice
Black coffee
Rainwater
Urine
Saliva
Pure water
Human blood, tears
Seawater
9
10
Milk of magnesia
Household ammonia
Household
bleach
Oven cleaner
11
12
13
14
Slide 40
Buffers
The internal pH of most living cells must remain close to pH 7
Buffers are substances that minimize changes in concentrations of H+ and OH– in a solution
Most buffers consist of an acid-base pair that reversibly combines with H+
Slide 41
Threats to Water Quality on Earth
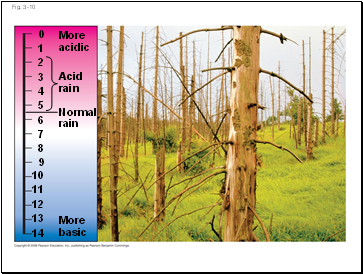
Acid precipitation refers to rain, snow, or fog with a pH lower than 5.6
Acid precipitation is caused mainly by the mixing of different pollutants with water in the air and can fall at some distance from the source of pollutants
Acid precipitation can damage life in lakes and streams
Effects of acid precipitation on soil chemistry are contributing to the decline of some forests
Slide 42
Fig. 3-10
More
acidic
0
Acid
rain
Acid
rain
Normal
rain
More
basic
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Slide 43
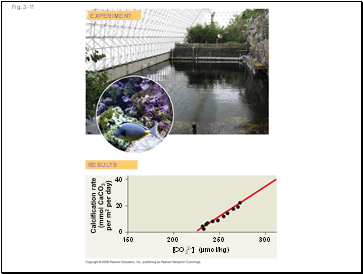
Human activities such as burning fossil fuels threaten water quality
CO2 is released by fossil fuel combustion and contributes to:
A warming of earth called the “greenhouse” effect
Acidification of the oceans; this leads to a decrease in the ability of corals to form calcified reefs
Slide 44
Fig. 3-11
EXPERIMENT
RESULTS
Calcification rate
(mmol CaCO3
per m2 per day)
[CO32–] (µmol/kg)
Contents
- The Molecule That Supports All of Life
- Cohesion
- Moderation of Temperature
- Insulation of Bodies of Water by Floating Ice
- The Solvent of Life
- Effects of Changes in pH
- Threats to Water Quality on Earth
Last added presentations
- Solar Thermal Energy
- Newton’s Laws of Motion
- Magnetic field uses sound waves to ignite sun's ring of fire
- Sound
- Gravitation
- Radioactivity and Nuclear Reactions
- Simulation at NASA for the Space Radiation Effort