Water and the Fitness of the EnvironmentPage
2
2
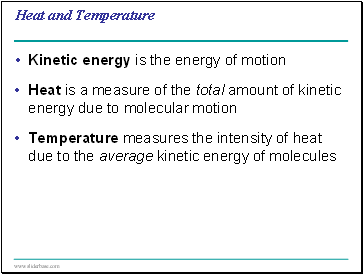
Heat and Temperature
Kinetic energy is the energy of motion
Heat is a measure of the total amount of kinetic energy due to molecular motion
Temperature measures the intensity of heat due to the average kinetic energy of molecules
Slide 13
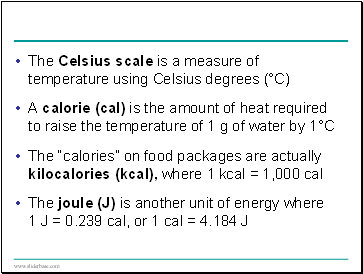
The Celsius scale is a measure of temperature using Celsius degrees (°C)
A calorie (cal) is the amount of heat required to raise the temperature of 1 g of water by 1°C
The “calories” on food packages are actually kilocalories (kcal), where 1 kcal = 1,000 cal
The joule (J) is another unit of energy where 1 J = 0.239 cal, or 1 cal = 4.184 J
Slide 14
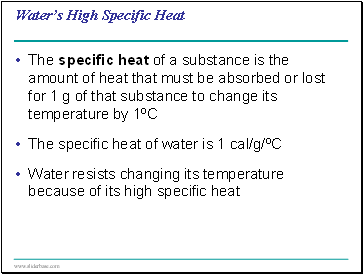
Water’s High Specific Heat
The specific heat of a substance is the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1ºC
The specific heat of water is 1 cal/g/ºC
Water resists changing its temperature because of its high specific heat
Slide 15
Water’s high specific heat can be traced to hydrogen bonding
Heat is absorbed when hydrogen bonds break
Heat is released when hydrogen bonds form
The high specific heat of water minimizes temperature fluctuations to within limits that permit life
Slide 16
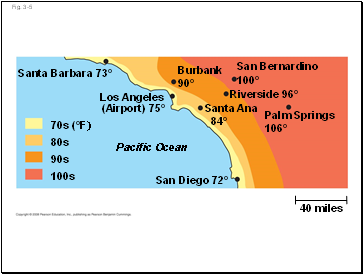
Fig. 3-5
San Diego 72°
40 miles
Pacific Ocean
70s (°F)
80s
90s
100s
Santa Barbara 73°
Los Angeles
(Airport) 75°
Burbank
90°
San Bernardino
100°
Riverside 96°
Santa Ana
84°
Palm Springs
106°
Slide 17
Evaporative Cooling
Evaporation is transformation of a substance from liquid to gas
Heat of vaporization is the heat a liquid must absorb for 1 g to be converted to gas
As a liquid evaporates, its remaining surface cools, a process called evaporative cooling
Evaporative cooling of water helps stabilize temperatures in organisms and bodies of water
Slide 18
Insulation of Bodies of Water by Floating Ice
Ice floats in liquid water because hydrogen bonds in ice are more “ordered,” making ice less dense
Water reaches its greatest density at 4°C
If ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth
Slide 19
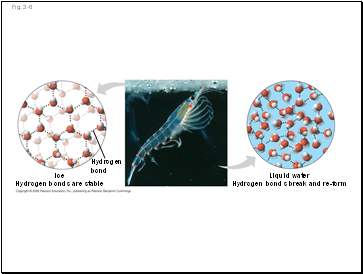
Fig. 3-6
Hydrogen
bond
Liquid water
Hydrogen bonds break and re-form
Contents
- The Molecule That Supports All of Life
- Cohesion
- Moderation of Temperature
- Insulation of Bodies of Water by Floating Ice
- The Solvent of Life
- Effects of Changes in pH
- Threats to Water Quality on Earth
Last added presentations
- Heat-Energy on the Move
- Radioactivity and Nuclear Reactions
- Radiation
- Buoyancy
- Static and Kinetic Friction
- Motion
- Sound