Water and the Fitness of the EnvironmentPage
3
3
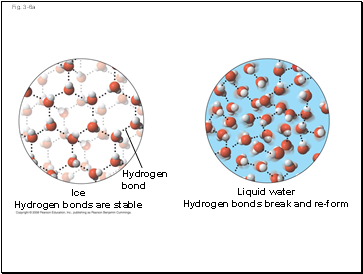
Ice
Hydrogen bonds are stable
Slide 20
Fig. 3-6a
Hydrogen
bond
Liquid water
Hydrogen bonds break and re-form
Ice
Hydrogen bonds are stable
Slide 21
The Solvent of Life
A solution is a liquid that is a homogeneous mixture of substances
A solvent is the dissolving agent of a solution
The solute is the substance that is dissolved
An aqueous solution is one in which water is the solvent
Slide 22
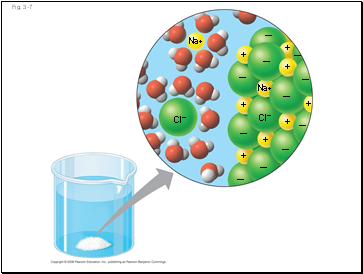
Water is a versatile solvent due to its polarity, which allows it to form hydrogen bonds easily
When an ionic compound is dissolved in water, each ion is surrounded by a sphere of water molecules called a hydration shell
Slide 23
Fig. 3-7
Cl–
Na
Cl–
+
+
+
+
+
+
+
+
–
–
–
–
–
–
–
–
Na
+
–
–
–
+
Slide 24
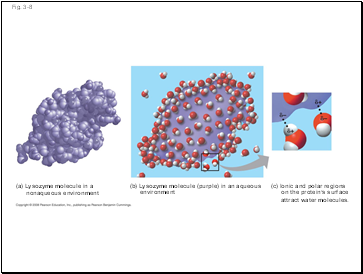
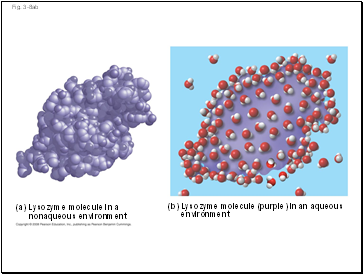
Water can also dissolve compounds made of nonionic polar molecules
Even large polar molecules such as proteins can dissolve in water if they have ionic and polar regions
Slide 25
Fig. 3-8
(a) Lysozyme molecule in a
nonaqueous environment
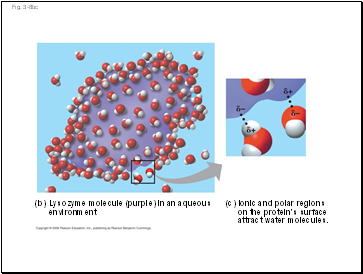
(b) Lysozyme molecule (purple) in an aqueous
environment
(c) Ionic and polar regions
on the protein’s surface
attract water molecules.
Slide 26
Fig. 3-8ab
(b) Lysozyme molecule (purple) in an aqueous
environment
(a) Lysozyme molecule in a
nonaqueous environment
Slide 27
Fig. 3-8bc
(c) Ionic and polar regions
on the protein’s surface
attract water molecules.
(b) Lysozyme molecule (purple) in an aqueous
environment
Slide 28
Hydrophilic and Hydrophobic Substances
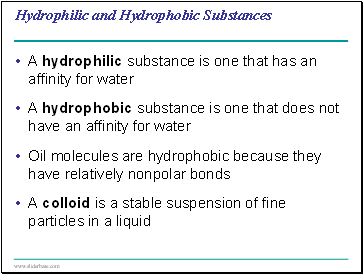
A hydrophilic substance is one that has an affinity for water
A hydrophobic substance is one that does not have an affinity for water
Oil molecules are hydrophobic because they have relatively nonpolar bonds
A colloid is a stable suspension of fine particles in a liquid
Slide 29
Solute Concentration in Aqueous Solutions
Most biochemical reactions occur in water
Contents
- The Molecule That Supports All of Life
- Cohesion
- Moderation of Temperature
- Insulation of Bodies of Water by Floating Ice
- The Solvent of Life
- Effects of Changes in pH
- Threats to Water Quality on Earth
Last added presentations
- Sound
- Static and Kinetic Friction
- Radioactivity and Nuclear Reactions
- Newton's laws of motion
- Newton’s law of universal gravitation
- Buoyancy
- Waves & Sound