Water and the Fitness of the EnvironmentPage
6
6
150
200
250
300
0
20
40
Slide 45
Fig. 3-11a
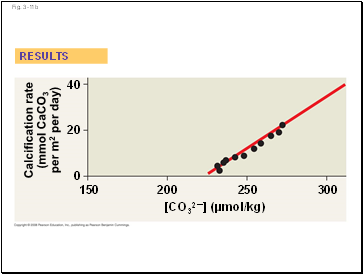
EXPERIMENT
Slide 46
Fig. 3-11b
Calcification rate
(mmol CaCO3
per m2 per day)
40
20
0
300
150
200
250
[CO32–] (µmol/kg)
RESULTS
Slide 47
Fig. 3-UN3
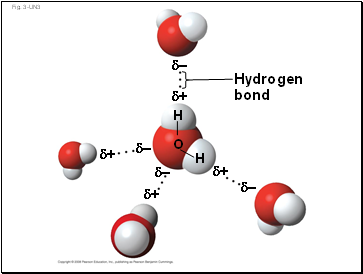
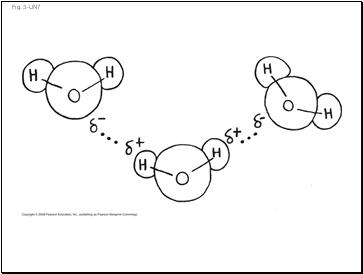
Hydrogen
bond
–
+
+
+
+
–
–
–
H
H
O
Slide 48
Fig. 3-UN4
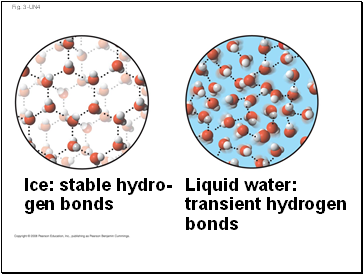
Liquid water:
transient hydrogen
bonds
Ice: stable hydro-
gen bonds
Slide 49
Fig. 3-UN5
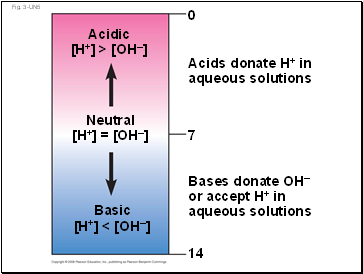
Bases donate OH–
or accept H+ in
aqueous solutions
Acids donate H+ in
aqueous solutions
Acidic
[H+] > [OH–]
Neutral
[H+] = [OH–]
Basic
[H+] < [OH–]
14
7
0
Slide 50
Fig. 3-UN6
Surface of Earth
Surface of Mars
Slide 51
Fig. 3-UN7
Slide 52
You should now be able to:
List and explain the four properties of water that emerge as a result of its ability to form hydrogen bonds
Distinguish between the following sets of terms: hydrophobic and hydrophilic substances; a solute, a solvent, and a solution
Define acid, base, and pH
Explain how buffers work
Contents
- The Molecule That Supports All of Life
- Cohesion
- Moderation of Temperature
- Insulation of Bodies of Water by Floating Ice
- The Solvent of Life
- Effects of Changes in pH
- Threats to Water Quality on Earth
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Sensory and Motor Mechanisms
- Newton’s laws of motion
- Gravitation
- Geophysical Concepts, Applications and Limitations
- Mechanics Lecture
- Health Physics