The SI SystemPage
1
1
Slide 1
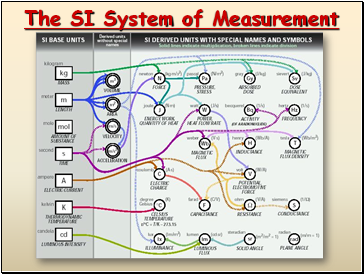
The SI System of Measurement
Slide 2
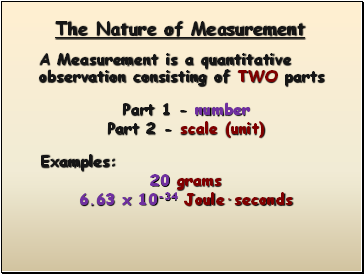
The Nature of Measurement
Part 1 - number
Part 2 - scale (unit)
Examples:
20 grams
6.63 x 10-34 Joule·seconds
A Measurement is a quantitative observation consisting of TWO parts
Slide 3
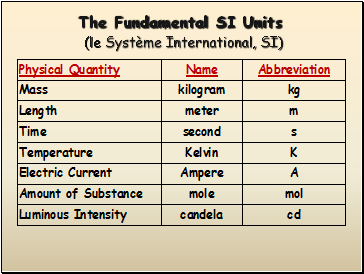
The Fundamental SI Units (le Système International, SI)
Slide 4
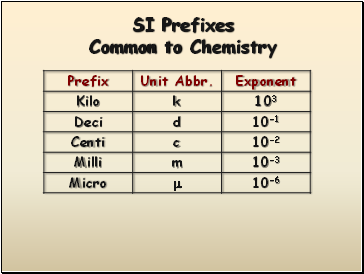
SI Prefixes Common to Chemistry
Slide 5
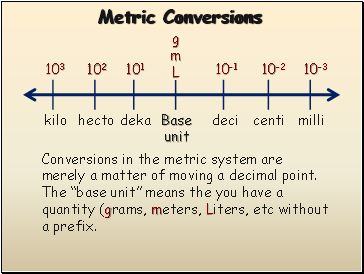
Metric Conversions
g
m
L
10-1
10-2
10-3
101
102
103
Base
unit
deci
centi
milli
deka
hecto
kilo
Conversions in the metric system are merely a matter of moving a decimal point. The “base unit” means the you have a quantity (grams, meters, Liters, etc without a prefix.
Slide 6
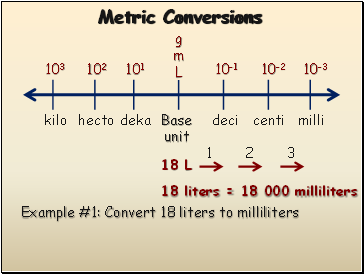
Metric Conversions
g
m
L
10-1
10-2
10-3
101
102
103
Base
unit
deci
centi
milli
deka
hecto
kilo
Example #1: Convert 18 liters to milliliters
18 L
1
2
3
18 liters = 18 000 milliliters
Slide 7
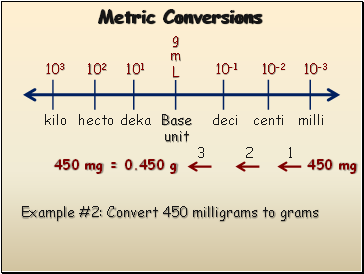
Metric Conversions
g
m
L
10-1
10-2
10-3
101
102
103
Base
unit
deci
centi
milli
deka
hecto
kilo
Example #2: Convert 450 milligrams to grams
1
2
3
450 mg
450 mg = 0.450 g
Slide 8
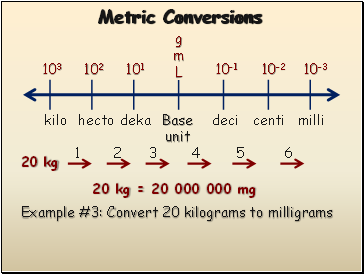
Metric Conversions
g
m
L
10-1
10-2
10-3
101
102
103
Base
unit
deci
centi
milli
deka
hecto
kilo
Example #3: Convert 20 kilograms to milligrams
20 kg
1
2
3
4
5
6
20 kg = 20 000 000 mg
Contents
Last added presentations
- Radiation
- Newton's laws of motion
- Buoyancy
- Solar Energy
- Waves & Sound
- Upcoming Classes
- Radioactivity and Nuclear Reactions