Atomic StructurePage
2
2
Slide 7
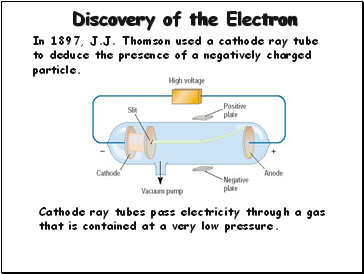
Discovery of the Electron
In 1897, J.J. Thomson used a cathode ray tube to deduce the presence of a negatively charged particle.
Cathode ray tubes pass electricity through a gas that is contained at a very low pressure.
Slide 8
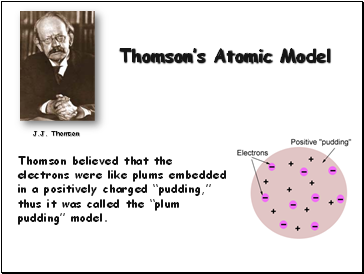
Thomson’s Atomic Model
Thomson believed that the electrons were like plums embedded in a positively charged “pudding,” thus it was called the “plum pudding” model.
J.J. Thomson
Slide 9
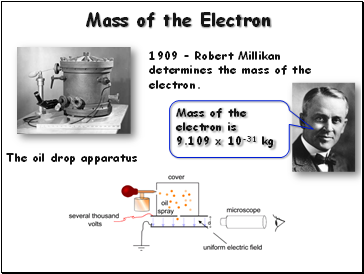
Mass of the Electron
1909 – Robert Millikan determines the mass of the electron.
The oil drop apparatus
Mass of the electron is
9.109 x 10-31 kg
Slide 10
Conclusions from the Study of the Electron
Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons.
Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons
Electrons have so little mass that atoms must contain other particles that account for most of the mass
Slide 11
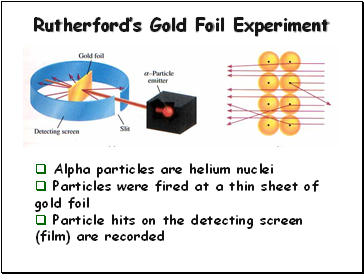
Rutherford’s Gold Foil Experiment
Alpha particles are helium nuclei
Particles were fired at a thin sheet of gold foil
Particle hits on the detecting screen (film) are recorded
Slide 12
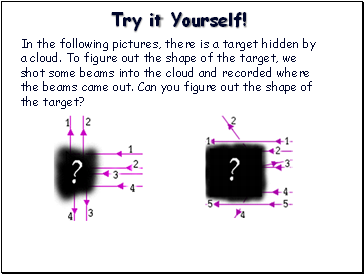
Try it Yourself!
In the following pictures, there is a target hidden by a cloud. To figure out the shape of the target, we shot some beams into the cloud and recorded where the beams came out. Can you figure out the shape of the target?
Slide 13
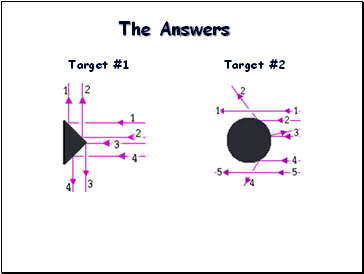
The Answers
Target #1
Target #2
Slide 14
Rutherford’s Findings
The nucleus is small
The nucleus is dense
The nucleus is positively charged
Most of the particles passed right through
A few particles were deflected
VERY FEW were greatly deflected
“Like howitzer shells bouncing off of tissue paper!”
Conclusions:
Slide 15
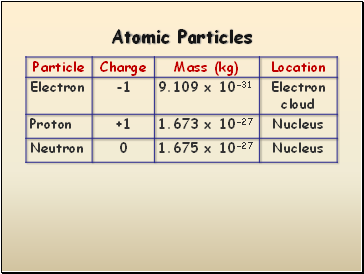
Atomic Particles
Slide 16
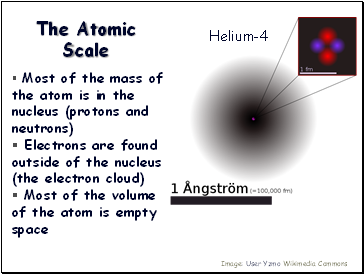
The Atomic Scale
Most of the mass of the atom is in the nucleus (protons and neutrons)
Electrons are found outside of the nucleus (the electron cloud)
Contents
- About Quarks…
- Isotopes
- Atomic Masses
- Atomic Number
- Discovery of the Electron
- Thomson’s Atomic Model
- Mass of the Electron
- Conclusions from the Study of the Electron
- Rutherford’s Gold Foil Experiment
- Rutherford’s Findings
- Atomic Particles
- The Atomic Scale
- Atomic Structure
- Chemistry Timeline
- Dalton’s Atomic Theory (1808)
- Modern Atomic Theory
Last added presentations
- Newton’s laws of motion
- Heat-Energy on the Move
- Newton's Laws
- Practical Applications of Solar Energy
- Thermal Energy
- Gravitation
- Simulation at NASA for the Space Radiation Effort