Atomic StructurePage
3
3
Most of the volume of the atom is empty space
Helium-4
Image: User Yzmo Wikimedia Commons.
Slide 17
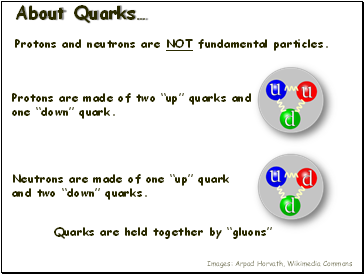
About Quarks…
Protons and neutrons are NOT fundamental particles.
Protons are made of two “up” quarks and one “down” quark.
Neutrons are made of one “up” quark and two “down” quarks.
Quarks are held together by “gluons”
Images: Arpad Horvath, Wikimedia Commons.
Slide 18
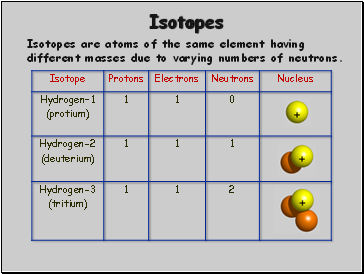
Isotopes
Isotopes are atoms of the same element having different masses due to varying numbers of neutrons.
Slide 19
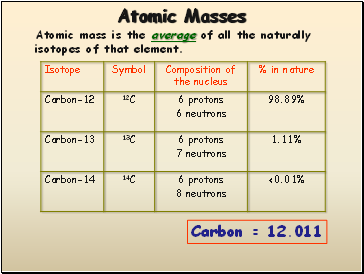
Atomic Masses
Atomic mass is the average of all the naturally isotopes of that element.
Carbon = 12.011
Slide 20
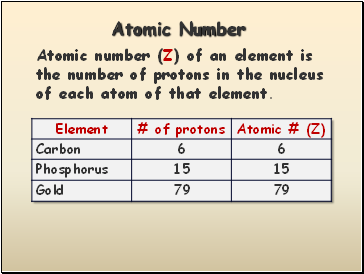
Atomic Number
Atomic number (Z) of an element is the number of protons in the nucleus of each atom of that element.
Slide 21
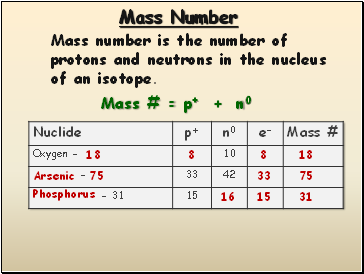
Mass Number
Mass number is the number of protons and neutrons in the nucleus of an isotope.
Mass # = p+ + n0
8
8
18
18
Arsenic
75
33
75
Phosphorus
15
31
16
Contents
- About Quarks…
- Isotopes
- Atomic Masses
- Atomic Number
- Discovery of the Electron
- Thomson’s Atomic Model
- Mass of the Electron
- Conclusions from the Study of the Electron
- Rutherford’s Gold Foil Experiment
- Rutherford’s Findings
- Atomic Particles
- The Atomic Scale
- Atomic Structure
- Chemistry Timeline
- Dalton’s Atomic Theory (1808)
- Modern Atomic Theory
Last added presentations
- Newton’s third law of motion
- Newton's Laws
- Newton’s Laws of Motion
- Waves & Sound
- Soil and Plant Nutrition
- Sound
- Solar Energy