Atoms and moleculesPage
2
2
The number of atoms or molecules in a mole is defined such that the mass of one mole of a substance, in grams, is exactly equal to the substance's atomic or molecular weight.
For example, the molecular weight of natural water is about 18.015, so one mole of water is about 18.015 grams.
Slide 11
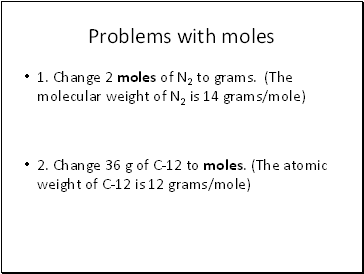
Problems with moles
1. Change 2 moles of N2 to grams. (The molecular weight of N2 is 14 grams/mole)
2. Change 36 g of C-12 to moles. (The atomic weight of C-12 is 12 grams/mole)
Go to page:
1 2
1 2
Contents
- More about atoms and molecules
- What is an atom mostly made of?
- The sizes of things in an atom
- The Rutherford Model of the Atom
- Probability
- Electron Orbitals
- Ions
- Avogadro Constant and Mole
- How to use a mole
- Problems with moles
Last added presentations
- Radiation
- Buoyancy
- Health Physics
- Ch 9 Nuclear Radiation
- Geophysical Concepts, Applications and Limitations
- Sound
- Direct heat utilization of geothermal energy
© 2010-2026 powerpoint presentations