Atoms, molecules and ionsPage
3
3
Al2O3
2.6
Al3+
O2-
CaBr2
Ca2+
Br-
Na2CO3
Na+
CO32-
Slide 26
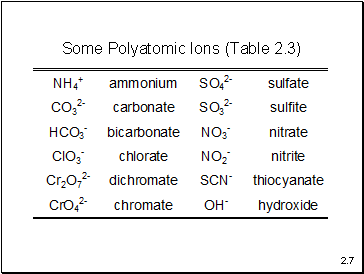
Some Polyatomic Ions (Table 2.3)
2.7
Slide 27
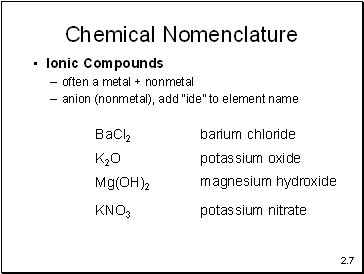
Chemical Nomenclature
Ionic Compounds
often a metal + nonmetal
anion (nonmetal), add “ide” to element name
BaCl2
barium chloride
K2O
potassium oxide
Mg(OH)2
magnesium hydroxide
KNO3
potassium nitrate
2.7
Slide 28
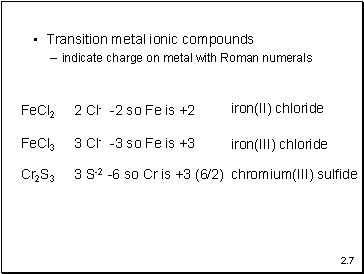
Transition metal ionic compounds
indicate charge on metal with Roman numerals
FeCl2
2 Cl- -2 so Fe is +2
iron(II) chloride
FeCl3
3 Cl- -3 so Fe is +3
iron(III) chloride
Cr2S3
3 S-2 -6 so Cr is +3 (6/2)
chromium(III) sulfide
2.7
Slide 29
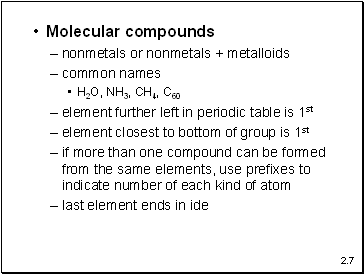
Molecular compounds
nonmetals or nonmetals + metalloids
common names
H2O, NH3, CH4, C60
element further left in periodic table is 1st
element closest to bottom of group is 1st
if more than one compound can be formed from the same elements, use prefixes to indicate number of each kind of atom
last element ends in ide
2.7
Slide 30
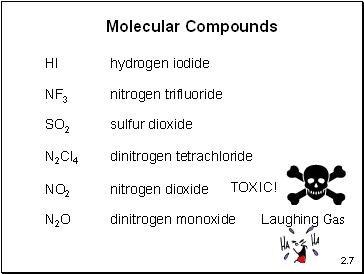
HI
hydrogen iodide
NF3
nitrogen trifluoride
SO2
sulfur dioxide
N2Cl4
dinitrogen tetrachloride
NO2
nitrogen dioxide
N2O
dinitrogen monoxide
Molecular Compounds
2.7
Slide 31
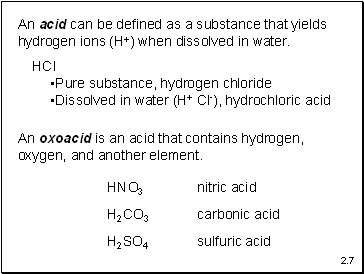
An acid can be defined as a substance that yields
hydrogen ions (H+) when dissolved in water.
HCl
Pure substance, hydrogen chloride
Dissolved in water (H+ Cl-), hydrochloric acid
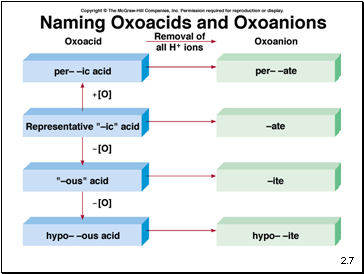
An oxoacid is an acid that contains hydrogen, oxygen, and another element.
2.7
Slide 32
2.7
Slide 33
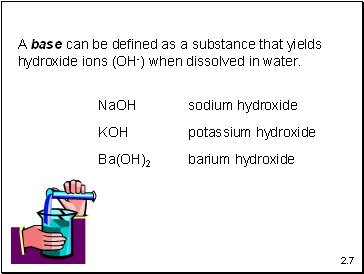
A base can be defined as a substance that yields
hydroxide ions (OH-) when dissolved in water.
2.7
Contents
- Atoms, Molecules and Ions
- Dalton’s Atomic Theory (1808)
- Chadwick’s Experiment (1932)
- Formula of Ionic Compounds
- Chemical Nomenclature
Last added presentations
- Radiation Safety and Operations
- Geophysical Concepts, Applications and Limitations
- Sensory and Motor Mechanisms
- Motion
- Direct heat utilization of geothermal energy
- Health Physics
- Radiation