ChemistryPage
4
4
Mr. Davis performs a chemical reaction for the class. Which of these does NOT show evidence that a chemical reaction has occurred ?
Change in color
Change in shape
Formation of gas
Formation of a precipitate
Slide 36
Which is the correct symbol for the element sodium?
S
Se
Cl
Na
Slide 37
Which statement is usually true about the electrical properties of metals?
Metals have high electrical resistance.
Lightweight metals are the best conductors.
Metals and plastics are both good insulators.
Metals are good electrical conductors.
Slide 38
Students poured different liquids to see how the liquids became layered. The denser liquids at the bottom, the lighter liquids at the top. What tool was used in this experiment?
a balance scale
a thermometer
a magnet
a graduated cylinder
Slide 39
Tom places four objects in a tank of water. He makes the following observations. Which statement is correct based on Tom's observations?
The rock and the chalk have a density greater than water.
The rock and the chalk have a density less than water.
The cork and the can of soda have a density equal to water.
The cork and the can of soda have a density greater than water
Slide 40
The chemical symbol Al represents which metal on the periodic table?
arsenic
antimony
aurum
aluminum
Slide 41
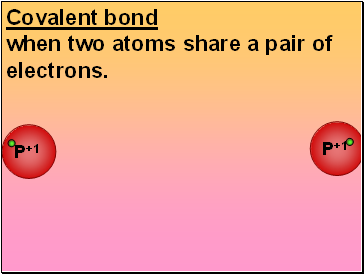
Covalent bond
P+1
when two atoms share a pair of electrons.
P+1
Slide 42
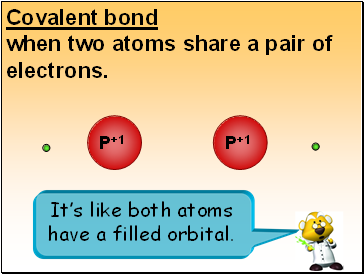
Covalent bond
when two atoms share a pair of electrons.
P+1
P+1
It’s like both atoms have a filled orbital.
Slide 43
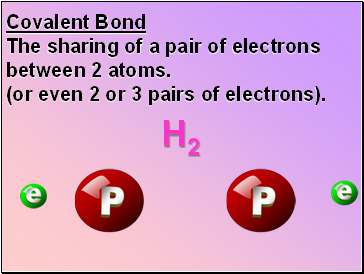
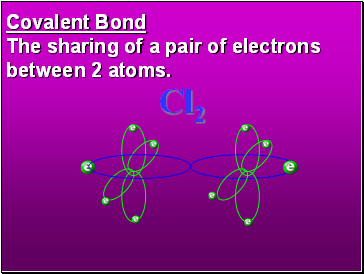
Covalent Bond
The sharing of a pair of electrons between 2 atoms.
(or even 2 or 3 pairs of electrons).
H2
Slide 44
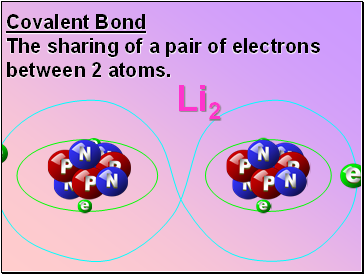
Covalent Bond
The sharing of a pair of electrons between 2 atoms.
Li2
Slide 45
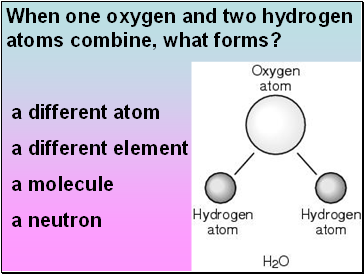
When one oxygen and two hydrogen atoms combine, what forms?
a different atom
a different element
a molecule
a neutron
Slide 46
Contents
- Reaction Types
- Chemical Bonds
- Ionic bond
- Cations
- Anions
- Which is a metric unit for density?
- When a gas forms a liquid, which process is taking place?
- Which unit correctly describes density?
- Based on the melting points shown in the table, which material would still be a solid at 400°C?
- A chemical change for a piece of metal would be
- Which symbolizes a molecule of a compound?
- Putting sand and salt together makes
- Plastic, wood, and iron are all made up of
- An atom is to an element, as a molecule is to a
- Which is the correct symbol for the element sodium?
- Covalent bond
- Reactivity
- Calorimeter
- Combustibility
- Biochemicals
- Sugars
- Wawa
Last added presentations
- Solar Thermal Energy
- Gravitation
- Newton’s Laws of Motion
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Sensory and Motor Mechanisms
- Radiation
- The Effects of Radiation on Living Things