Gas Temperature Volume and PressurePage
1
1
Slide 1
Gas Temperature, Volume, and Pressure
This tool can measure the temperature, volume, and pressure of a gas
Slide 2
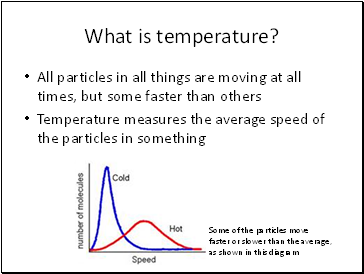
What is temperature?
All particles in all things are moving at all times, but some faster than others
Temperature measures the average speed of the particles in something
Some of the particles move faster or slower than the average, as shown in this diagram
Slide 3
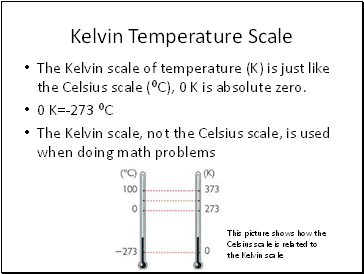
Kelvin Temperature Scale
The Kelvin scale of temperature (K) is just like the Celsius scale (⁰C), 0 K is absolute zero.
0 K=-273 ⁰C
The Kelvin scale, not the Celsius scale, is used when doing math problems
This picture shows how the Celsius scale is related to the Kelvin scale
Slide 4
Absolute Zero Temperature
Absolute zero (0 K) is the coldest possible temperature. It is the temperature at which all movement stops
Scientists have made temperatures very close to absolute zero, but it is thought that absolute zero can never be reached
Absolute Zero is much colder than this
Slide 5
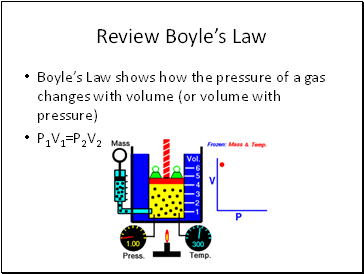
Review Boyle’s Law
Boyle’s Law shows how the pressure of a gas changes with volume (or volume with pressure)
P1V1=P2V2
Slide 6
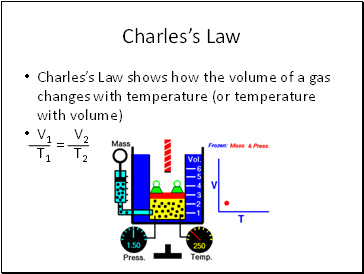
Charles’s Law
Charles’s Law shows how the volume of a gas changes with temperature (or temperature with volume)
V1 V2
T1 T2
Slide 7
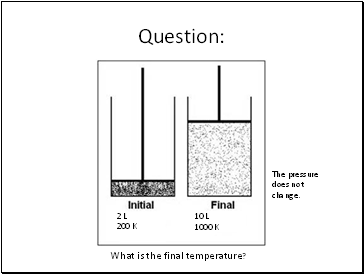
Question:
2 L
200 K
10 L
What is the final temperature?
1000 K
The pressure does not change.
Slide 8
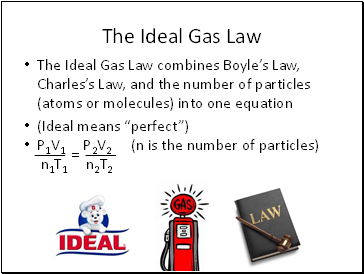
The Ideal Gas Law
The Ideal Gas Law combines Boyle’s Law, Charles’s Law, and the number of particles (atoms or molecules) into one equation
(Ideal means “perfect”)
P1V1 P2V2 (n is the number of particles)
n1T1 n2T2
Slide 9
Ideal Gas Law Assumptions
An assumption is something taken as true in a certain situation
The Ideal Gas Law assumes a few things about the particles in a gas
These assumptions are usually very close to being true, but are never completely true
That’s why it’s called the “Ideal” Gas Law instead of the “Real” Gas Law
Slide 10
1 2
Contents
- What is temperature?
- Kelvin Temperature Scale
- Absolute Zero Temperature
- Review Boyle’s Law
- Charles’s Law
- The Ideal Gas Law
- Ideal Gas Law Assumptions
Last added presentations
- Health Physics
- Thermal Energy
- Radiation Safety and Operations
- Newton’s Laws of Motion
- Geophysical Concepts, Applications and Limitations
- Newton's Laws
- Space Radiation