Earth MaterialsPage
3
3
We ‘Share’ the Oil field with others in Europe, having a claim to the fields
Slide 18
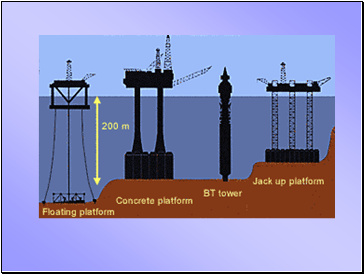
Oil platforms in the North Sea are huge
They stand on tall legs anchored to the sea bed
Slide 19
Slide 20
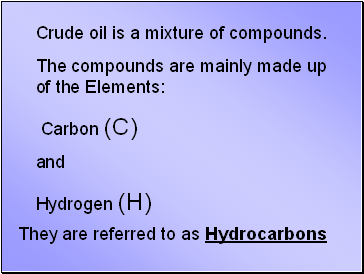
Crude oil is a mixture of compounds.
The compounds are mainly made up of the Elements:
Carbon (C)
and
Hydrogen (H)
They are referred to as Hydrocarbons
Slide 21
The Chemical and Physical properties of Hydrocarbon Molecules in the mixture are unchanged by the fact that they are in a mixture:
This means that each compound in the mixture will boils at its own, unique, boiling point.
This helps us to separate the mixture
Slide 22
Since it is a mixture, crude Oil found in one location may be different to that found in another.
Slide 23
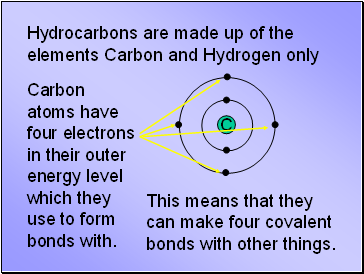
Hydrocarbons are made up of the elements Carbon and Hydrogen only
Carbon atoms have four electrons in their outer energy level which they use to form bonds with.
This means that they can make four covalent bonds with other things.
Slide 24
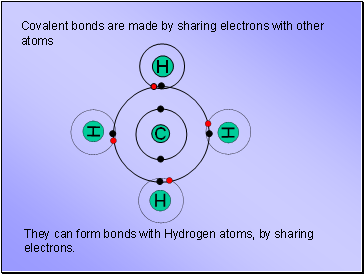
Covalent bonds are made by sharing electrons with other atoms
They can form bonds with Hydrogen atoms, by sharing electrons.
Slide 25
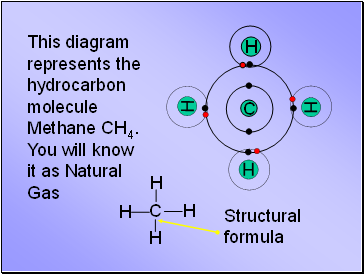
This diagram represents the hydrocarbon molecule Methane CH4. You will know it as Natural Gas
Structural formula
Slide 26
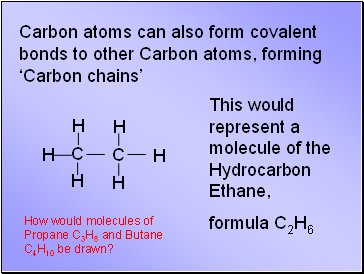
Carbon atoms can also form covalent bonds to other Carbon atoms, forming ‘Carbon chains’
This would represent a molecule of the Hydrocarbon Ethane,
formula C2H6
H
How would molecules of Propane C3H8 and Butane C4H10 be drawn?
Slide 27
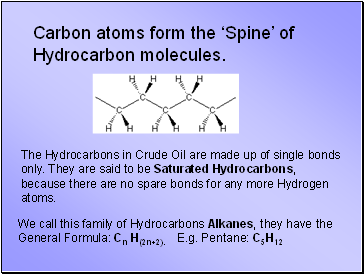
Carbon atoms form the ‘Spine’ of Hydrocarbon molecules.
The Hydrocarbons in Crude Oil are made up of single bonds only. They are said to be Saturated Hydrocarbons, because there are no spare bonds for any more Hydrogen atoms.
We call this family of Hydrocarbons Alkanes, they have the General Formula: Cn H(2n+2). E.g. Pentane: C5H12
Slide 28
The number of carbon atoms in the Hydrocarbon molecules in Crude Oil varies from 1carbon to over 70 carbons atoms.
Contents
- Earth Materials. Module 06
- Limestone
- Reminder:
- Cement
- Glass
- The Oil is often found in porous rock
- Reminder!
- More about Catalytic Cracking
- A Test for Unsaturated Hydrocarbons
- Why bother about Alkenes?
- Addition Polymerisation
- Ethene to Poly(ethene)
- Representing Polymerisation
- Some uses of Plastics
- Problems with Plastics
- Why not recycle plastics?
- Why not burn them then?
- The need for a balanced solution
Last added presentations
- Waves & Sound
- The Effects of Radiation on Living Things
- Buoyancy
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Sound
- Newton’s Laws of Motion
- Practical Applications of Solar Energy