Acids, Bases, & SaltsPage
2
2
Conjugate acid- compound formed when an base gains a hydrogen ion.
Conjugate base – compound formed when an acid loses a hydrogen ion.
Slide 13
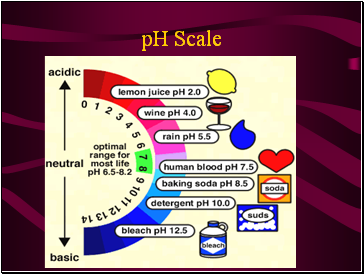
pH Scale
Slide 14
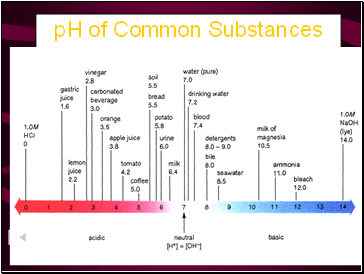
pH of Common Substances
Timberlake, Chemistry 7th Edition, page 335
Slide 15
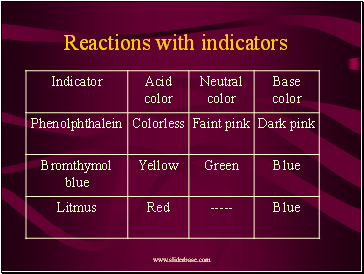
Reactions with indicators
Slide 16

pH paper
pH paper changes color to indicate a specific pH value.
Slide 17

Buffers
A buffer is a solution that resists changes in pH when small amounts of acids and bases are added.
Slide 18

Situations in which pH is controlled
“Heartburn”
Planting vegetables and flowers
Fish Tanks and Ponds
Blood
Swimming pools
Slide 19

Acids and Bases in Solution
HCl + H20 H3O + + Cl- (more hydronium ions, more acidic)
NaOH in water Na+ + OH- (more hydroxide ions, more basic)
NaOH + HCl NaCl + HOH Acid + Base yields type of salt and water
NH3 + H20 NH4+ + OH- ammonia gas + water yields ammonium and hydroxide ions
Slide 20

Acid Rain
Pollution in the air (sulfur dioxide, carbon dioxide, nitrogen dioxide) combines with water to form various acids.
.
Slide 21

Rapid changes in pH can kill fish and other organisms in lakes and streams.
Soil pH is affected and can kill plants and create sinkholes
Slide 22

Slide 23

Slide 24

Slide 25

What is a SALT?
A salt is a neutral substance produced from the reaction of an acid and a base.
Composed of the negative ion of an acid and the positive ion of a base.
One of the products of a Neutralization Reaction
Examples: KCl, MgSO4, Na3PO4
Slide 26
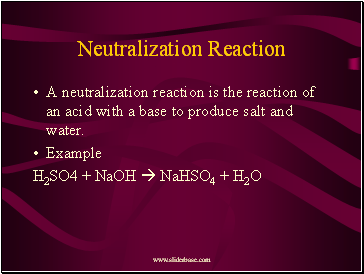
Neutralization Reaction
A neutralization reaction is the reaction of an acid with a base to produce salt and water.
Contents
- What is an ACID?
- Acids Generate Ions
- Weak vs. Strong Acids
- Common Acids
- What is a BASE?
- Weak vs. Strong Bases
- Common Bases
- Types of Acids and Bases
- pH Scale
- pH paper
- Buffers
- Situations in which pH is controlled
- Acids and Bases in Solution
- Acid Rain
- What is a SALT?
- Neutralization Reaction
- Digestion and pH
- pH in the Digestive System
Last added presentations
- Ch 9 Nuclear Radiation
- Gravitation
- Magnetic field uses sound waves to ignite sun's ring of fire
- Newton's laws of motion
- The Effects of Radiation on Living Things
- Newton’s third law of motion
- Mechanics Lecture
