Periodictable - QuestionsPage
4
4
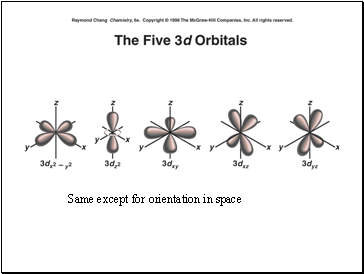
Same except for orientation in space
Slide 30
Same except for orientation in space
Slide 31
Electron spin
Each orbital can hold at most two electrons. Electrons also have spin (turning on an axis) and have magnetic properties (deflected in magnetic field). Electrons in the same orbital must have opposite spins. If they have opposite spins the electrons are said to be paired.
Slide 32
What to do with all this info?
Rules for writing electron configuration:
1. The no. of electrons in neutral atom = atomic no. (no. of protons)
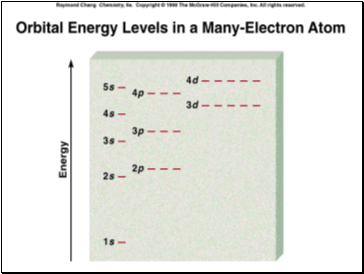
2. Fill the lowest energy sublevel completely, then the next lowest, etc.
3. No more than two electrons can be placed in a single orbital. The electrons have opposite spins in the same orbital. (2 electrons in s, 6 in p, 10 in d, 14 in f)
Slide 33
4. For n=1,
For n =2
For n=3,
For n=4,
Remember the order of filling as follows:
Slide 34
Slide 35
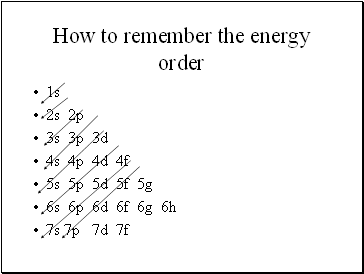
How to remember the energy order
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f 5g
6s 6p 6d 6f 6g 6h
7s 7p 7d 7f
Slide 36
Letís do some electron configurations
Slide 37
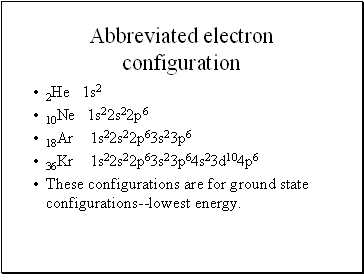
Abbreviated electron configuration
2He 1s2
10Ne 1s22s22p6
18Ar 1s22s22p63s23p6
36Kr 1s22s22p63s23p64s23d104p6
These configurations are for ground state configurations--lowest energy.
Slide 38
Valence electrons
, p 59
Valence electrons are the electrons located in the _ orbitals and are the ones involved in forming chemical bonds. The valence electrons have the largest _ value for the A elements.
For representative elements the number of valence electrons in an atom =
Slide 39
Donít worry about inner core of electrons (smaller n) since these are filled levels and donít enter into bond formation ( for A groups)
Slide 40
Valence electron configuration for A groups
Group IA
Group IIA
Group IIIA
Group IVA
Group VA
Group VIA
Group VIIA
Group VIIIA
Contents
- Elements, atoms, ions, and the periodic table
- The periodic law and the periodic table
- Early periodic tables
- Modern periodic table
- Metals and nonmetals
- More info from periodic table
- Electron arrangement and the periodic table
- Principal energy levels (shells)
- Sublevels
- Orbitals
- Electron spin
- What to do with all this info?
- Abbreviated electron configuration
- Valence electrons
- Valence electron configuration for A groups
- The octet rule
- Transition metal cations
- Whatís the ion formed by
- Isoelectronic
- Trends in the periodic table
- Size across a period
- Ion size
- Ionization energy
- Trends in ionization energy
- Electron affinity
- Trends in electron affinities
Last added presentations
- Newtonís law of universal gravitation
- Radiation Safety and Operations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Motion
- Upcoming Classes
- Radiation
- Magnetic field uses sound waves to ignite sun's ring of fire