Acid, bases and saltsPage
6
6
KHSO4(aq) + KOH ================ K2SO4(aq) + H+O(l)
Slide 34
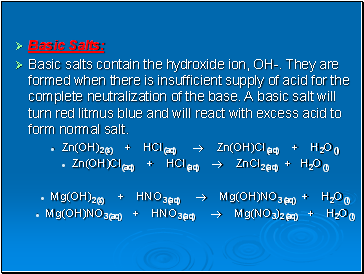
Basic Salts:
Basic salts contain the hydroxide ion, OH-. They are formed when there is insufficient supply of acid for the complete neutralization of the base. A basic salt will turn red litmus blue and will react with excess acid to form normal salt.
Zn(OH)2(s) + HCl(aq) Zn(OH)Cl(aq) + H2O(l)
Zn(OH)Cl(aq) + HCl(aq) ZnCl2(aq) + H2O(l)
Mg(OH)2(s) + HNO3(aq) Mg(OH)NO3(aq) + H2O(l)
Mg(OH)NO3(aq) + HNO3(aq) Mg(NO3)2(aq) + H2O(l)
Slide 35
Hydrated & anhydrous salts
Hydrated Salt: Salt that contains Water of Crystallization is called Hydrated Salt e.g. CuSO4.5H2O, Na2CO3.10H2O.
Anhydrous Salt: Salt with out Water of Crystallization is called Anhydrous Salt. e.g. CuSO4, Na2CO3
Slide 36
Slide 37
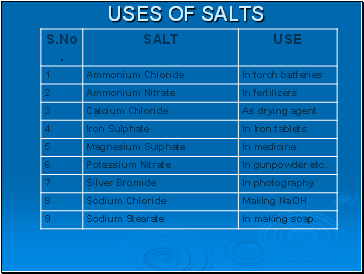
Uses of salts
Slide 38
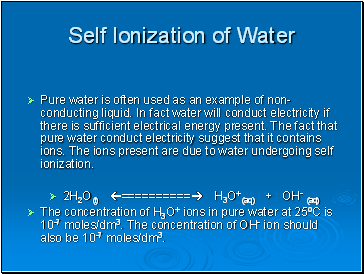
Self Ionization of Water
Pure water is often used as an example of non- conducting liquid. In fact water will conduct electricity if there is sufficient electrical energy present. The fact that pure water conduct electricity suggest that it contains ions. The ions present are due to water undergoing self ionization.
2H2O(l) ========== H3O+(aq) + OH- (aq)
The concentration of H3O+ ions in pure water at 25oC is 10-7 moles/dm3. The concentration of OH- ion should also be 10-7 moles/dm3.
Slide 39
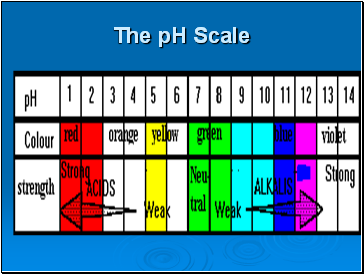
The pH Scale
Slide 40
The pH scale is a measure of the relative acidity or alkalinity of a solution.
It is defined as negative log of H+ ion concentration.
pH = -log [H+]
Water is a neutral liquid with a pH of 7 (green). When a substance dissolves in water it forms an aqueous solution that may be acidic, neutral or alkaline.
Acidic solutions have a pH of less than 7, and the lower the number, the stronger the acid is
Neutral solutions have a pH of 7. These are quite often solutions of salts, which are themselves formed from neutralizing acids and bases.
Alkaline solutions have a pH of over 7 and the higher the pH the stronger is the alkali. Weak alkalis like ammonia give a pH of 10-11 but strong alkalis like sodium hydroxide give a pH of 13-14.
Slide 41
pH
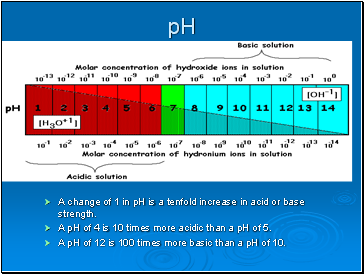
A change of 1 in pH is a tenfold increase in acid or base strength.
Contents
- Acids, Bases & Salts
- Terms
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- Formation of Hydronium ion( H30+).
- Uses of Acids
- Chemical Properties of Bases
- Chemical Properties of Bases
- Types of Oxides
- Periodic trends in oxides
- Salts
- Methods of making Soluble Salts
- Making Insoluble Salts
- Precipitation reaction
- Types of Salts
- Hydrated & anhydrous salts
- Uses of salts
- Self Ionization of Water
- The pH Scale
- Indicators.
- pH Graph
- Ionic equations
- Scheme for ionic equation
Last added presentations
- Direct heat utilization of geothermal energy
- Simulation at NASA for the Space Radiation Effort
- Sensory and Motor Mechanisms
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Practical Applications of Solar Energy
- Static and Kinetic Friction
- Magnetic field uses sound waves to ignite sun's ring of fire