Acids and Bases. Arrhenius Acids and BasesPage
2
2
HNO3 Ca(OH)2
H2SO4 Ba(OH)2
HClO4 Sr(OH)2
HNO3 → H+ + NO3-
Slide 11
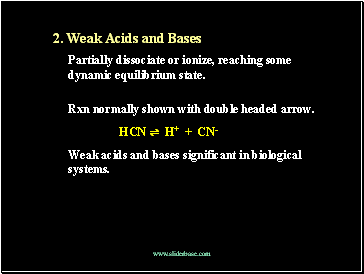
2. Weak Acids and Bases
Partially dissociate or ionize, reaching some dynamic equilibrium state.
Rxn normally shown with double headed arrow.
HCN ⇌ H+ + CN-
Weak acids and bases significant in biological systems.
Slide 12
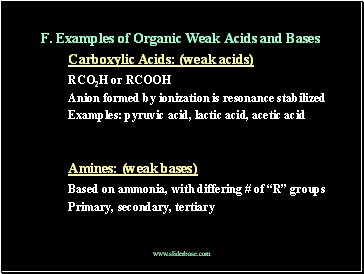
F. Examples of Organic Weak Acids and Bases
Carboxylic Acids: (weak acids)
RCO2H or RCOOH
Anion formed by ionization is resonance stabilized
Examples: pyruvic acid, lactic acid, acetic acid
Amines: (weak bases)
Based on ammonia, with differing # of “R” groups
Primary, secondary, tertiary
Slide 13

G. Polyprotic Acids and Bases
Polyprotic acids can donate more than one proton sequentially.
H2SO4
H3PO4
H2S
Polyprotic bases can accept more than one proton sequentially.
SO42-
PO43-
S2-
Slide 14
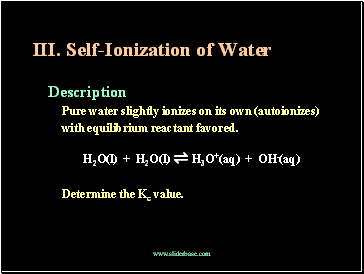
Self-Ionization of Water
Description
Pure water slightly ionizes on its own (autoionizes)
with equilibrium reactant favored.
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH-(aq)
Determine the Kc value.
Slide 15
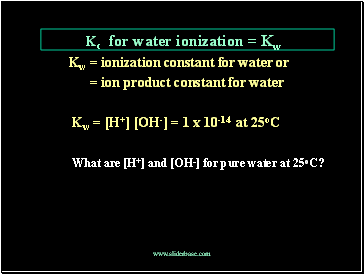
Kc for water ionization = Kw
Kw = ionization constant for water or
= ion product constant for water
Kw = [H+] [OH-] = 1 x 10-14 at 25oC
What are [H+] and [OH-] for pure water at 25oC?
Slide 16
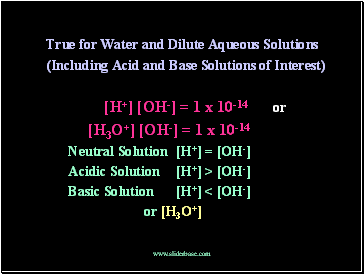
True for Water and Dilute Aqueous Solutions
(Including Acid and Base Solutions of Interest)
[H+] [OH-] = 1 x 10-14 or
[H3O+] [OH-] = 1 x 10-14
Neutral Solution [H+] = [OH-]
Acidic Solution [H+] > [OH-]
Basic Solution [H+] < [OH-]
or [H3O+]
Slide 17
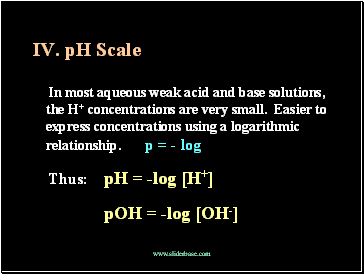
pH Scale
In most aqueous weak acid and base solutions, the H+ concentrations are very small. Easier to express concentrations using a logarithic relationship. p = - log
Thus: pH = -log [H+]
pOH = -log [OH-]
Slide 18
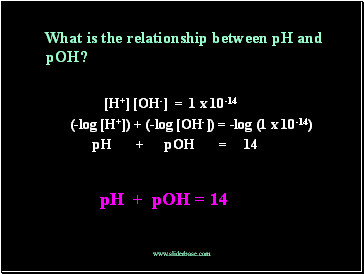
What is the relationship between pH and pOH?
[H+] [OH-] = 1 x 10-14
(-log [H+]) + (-log [OH-]) = -log (1 x 10-14)
pH + pOH = 14
pH + pOH = 14
Slide 19
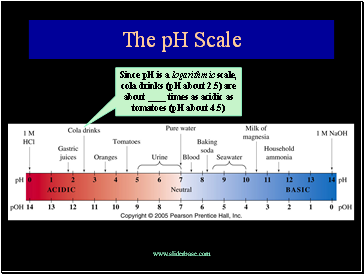
The pH Scale
Since pH is a logarithic scale, cola drinks (pH about 2.5) are about times as acidic as tomatoes (pH about 4.5)
Contents
- Arrhenius Acids and Bases
- Bronsted-Lowry Acids & Bases
- Self-Ionization of Water
- pH Scale
- Strong Acids and Bases
- Weak Acid Equilibrium Rxns
- Weak Base Equilibrium Rxns
- Relationship of Ka and Kb
- Acid-Base Reactions of Salts (Ions as Acids and Bases)
- Lewis Acids and Bases
- Acid Rain
Last added presentations
- Upcoming Classes
- Magnetic field uses sound waves to ignite sun's ring of fire
- Thermal Energy
- Health Physics
- Geophysical Concepts, Applications and Limitations
- Newton’s law of universal gravitation
- Radiation Safety and Operations
