Acids, Bases & SaltsPage
7
7
جمعرات، 28 ربیع الثانی، 1438
Slide 29
Topic 10: ACIDS, BASES & SALTS
29
جمعرات، 28 ربیع الثانی، 1438
Slide 30

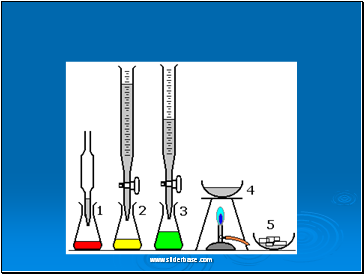
Making Insoluble Salts
Topic 10: ACIDS, BASES & SALTS
30
This involves mixing solutions of two soluble salts that between them contain the ions that make up the insoluble salt. It is made by two methods.
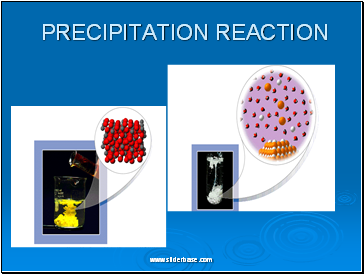
PRECIPITATION
BaCl2(aq) + MgSO4(aq) BaSO4(s) + MgCl2(aq)
DIRECT COMBINATION
Fe + S ---heat---- FeS
جمعرات، 28 ربیع الثانی، 1438
Slide 31
Topic 10: ACIDS, BASES & SALTS
31
PRECIPITATION REACTION
جمعرات، 28 ربیع الثانی، 1438
Slide 32
Types of Salts
Topic 10: ACIDS, BASES & SALTS
32
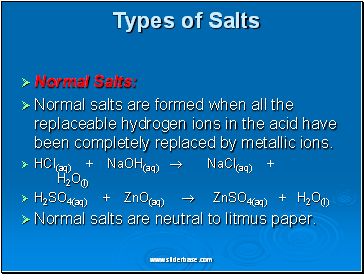
Normal Salts:
Normal salts are formed when all the replaceable hydrogen ions in the acid have been completely replaced by metallic ions.
HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l)
H2SO4(aq) + ZnO(aq) ZnSO4(aq) + H2O(l)
Normal salts are neutral to litmus paper.
جمعرات، 28 ربیع الثانی، 1438
Slide 33
Topic 10: ACIDS, BASES & SALTS
33
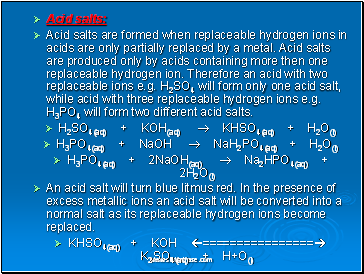
Acid salts:
Acid salts are formed when replaceable hydrogen ions in acids are only partially replaced by a metal. Acid salts are produced only by acids containing more then one replaceable hydrogen ion. Therefore an acid with two replaceable ions e.g. H2SO4 will form only one acid salt, while acid with three replaceable hydrogen ions e.g. H3PO4 will form two different acid salts.
H2SO4(aq) + KOH(aq) KHSO4(aq) + H2O(l)
H3PO4(aq) + NaOH NaH2PO4(aq) + H2O(l)
H3PO4(aq) + 2NaOH(aq) Na2HPO4(aq) + 2H2O(l)
An acid salt will turn blue litmus red. In the presence of excess metallic ions an acid salt will be converted into a normal salt as its replaceable hydrogen ions become replaced.
KHSO4(aq) + KOH ================ K2SO4(aq) + H+O(l)
جمعرات، 28 ربیع الثانی، 1438
Contents
- TERMS
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- USES OF ACIDS
- Chemical Properties of Bases
- TYPES OF OXIDES
- SALTS
- Methods of making Soluble Salts
- Making Insoluble Salts
- Types of Salts
- HYDRATED & ANHYDROUS SALTS
- Self Ionization of Water
- pH Graph
- IONIC EQUATIONS
Last added presentations
- Friction
- Sensory and Motor Mechanisms
- Static and Kinetic Friction
- Newton’s third law of motion
- Newton's laws of motion
- Solar Thermal Energy
- Heat-Energy on the Move
